|
Changes in growth and tropane alkaloid production in long-term
culture of hairy roots of Brugmansia candida
Patricia L. Marconi, Lorena M. Setten, Eugenio N. Cálcena, María
A. Alvarez and
Sandra I. Pitta-Alvarez
Fundación Pablo Cassará, Saladillo 2452 (C1440FFX), Ciudad
Autónoma de Buenos Aires, Argentina.
*Corresponding author; Sandra I. Pitta-Alvarez,
spitta@fundacioncassara.org.ar
Keywords:
hairy roots, tropane alkaloids, Brugmansia, hyoscyamine,
scopolamine
ABSTRACT
Hairy roots cultures, which are the result of infection with
Agrobacterium rhizogenes, present a number of advantages
over other in vitro cultures. One of the most important
ones is the genotypic and phenotypic stability of these cultures
over the years. Our group has been working with hairy roots of
Brugmansia candida, producer of the tropane
alkaloids scopolamine and hyoscyamine, widely used in medicine
as anticholinergics. Surprisingly, in our research, which
spanned 5 years of culture, we encountered a pronounced increase
in the production of scopolamine and a significant decrease in
growth. Contradictory results have been found in the literature,
where long-term stability as well as modifications in phenotypes
after several subcultures were reported. Since these metabolites
are involved in defense mechanisms and, in addition, in vitro
cultures represent in themselves a stressful situation, it could
be hypothesized that the whole biosynthetic pathway could be
up-regulated. The limitations to this accumulation could be
determined by negative feedback mechanisms or by the incapacity
of the roots to tolerate its toxicity. The hairy roots obtained
in our laboratory were not engineered to overexpress
hyoscyamine-6-β-hydroxydase (H6H), and therefore the changes
observed cannot be attributed to higher amounts of this enzyme.
However, further studies, particularly at the molecular level,
have to be initiated to determine if subculture of the roots
affect their genetic stability. Nevertheless, from the cited
data, it is clear that stability must be permanently controlled,
particularly if long-term industrial processes are entailed.
INTRODUCTION
Agrobacterium tumefaciens
and A. rhizogenes were discovered in the 1930´s, but it
was only in the late 1970´s that the particular mechanism of
action underlying the capacity of A. tumefaciens to
produce crown-gall tumor was elucidated (Chilton et al., 1997).
A. rhizogenes, while sharing many of the same
characteristics as its close relative, displays some important
differences. The main one is the induction of transformed
(hairy) roots at the site of infection (Chilton et al.,
1982). These two Gram negative bacteria have played a major role
in the history of plant tissue culture and molecular biology.
They allowed, for the first time, the establishment of in
vitro cultures of cells, tissues and organs in media devoid
of plant growth regulators (PGRs). At the same time, their role
as vectors to transfer genes into plant cells revolutionized
plant transformation (Wisniewski et al., 2002; Glaser and Matten,
2003; Christou et al., 2006).
In
the present work, we will focus on the use of hairy roots for
the production of plant secondary metabolites. The latter are
usually synthesized in low levels but play important roles in
plants (example: defense, attractants for pollinization, etc.).
Secondary metabolites are particularly important for mankind
because they are used in a variety of industries (Table I),
especially the pharmaceutical one. The advantages of hairy root
cultures are manifold. They can grow almost as fast as plant
cell suspensions, but maintaining a stable differentiated
phenotype. Many secondary products, among them tropane
alkaloids, are not expressed in undifferentiated cell cultures
efficiently because their synthesis is linked to root
differentiation. Consequently, hairy root cultures can express
many specific metabolic pathways, particularly secondary
metabolite ones, efficiently and similarly to roots in planta
( Hamill et al., 1986; Kamada et al., 1986; Flores et al, 1987;
Pitta-Alvarez and Giulietti, 1995). In some instances, hairy
roots can produce higher levels of the secondary metabolite
compared to the whole plant (Payne et al.,1987). Most
importantly, apparently they do not present the production
instability of plant suspension cultures. Hairy roots usually
have a long-term and stable production of secondary metabolites
(Flores et al., 1987) and they have been employed to produce a
large variety of secondary metabolites, among them tropane
alkaloids.
In
our laboratory, hairy roots of Brugmansia candida (Solanaceae)
were obtained for the production of the tropane alkaloids (‑)hyoscyamine
and, especially, scopolamine. Both are anticholinergic agents
widely used in medicine. However, scopolamine has a world demand
estimated to be 10 times greater than that of (‑)hyoscyamine and
atropine combined (Hashimoto et al., 1993). Figure 1 shows the
biosynthetic route of scopolamine. Hyoscyamine-6-β-hydroxylase
(H6H) is one of the key enzymes in the route and it is
involved in the hydroxylation and epoxidation of (‑)hyoscyamine
to render
Table I.
Secondary metabolites and their uses in various industries.
|
Industry |
Secondary metabolite |
Chemical structure |
Species |
Uses |
|
Pharmaceuticals |
Codeine
Quinine
Artemisinine
Scopolamine
Vincristine
Taxol |
Alkaloid
Alkaloid
Sesquiterpenic lactone
Alkaloid
Alkaloid
Diterpene |
Papaver somniferum
Cinchona ledgeriana
Artemisia annua
Datura stramonium
Brugmansia candida
Catharanthus roseus
Taxus sp. |
Cough syrups
Antimalarial
Antimalarial
Antispasmodic
Anti-leukemic
Anti-cancer |
|
Agrochemical |
Pyrethrin |
Terpene |
Chrysanthemum cinearifolium |
Insecticide |
|
Food |
Thaumatine |
Chalcone |
Cinchona ledgeriana |
Non-caloric sweetener |
|
Cosmetics |
Jasmine |
Essential oil |
Jasminum sp. |
Perfume |
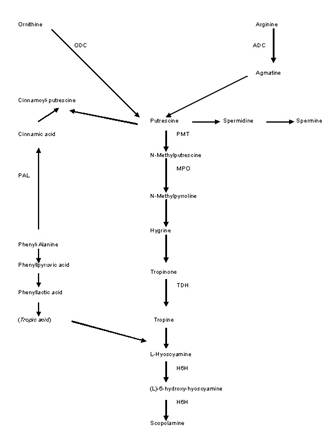
Figure
1.
Biosynthesis of hyoscyamine and scopolamine: ODC, ornithine
decarboxylase; ADC, arginine-decarboxylase; TDH, tropinone
dehydrogenase; H6H, hyoscyamine-6-β-hydroxylase.
scopolamine. H6H is localized in the pericycle of the root and is
particularly active in cultured roots, but it is absent in aerial
parts of the plant (Hashimoto et al.,1991; Matzuda et al., 1991).
As was
stated previously, one of the advantages of hairy roots is their
production stability, as opposed to plant cell cultures that are
subject to variations. However, the question remains as to the
long-term stability of transformed root cultures. In the literature
published so far, there are contradictory reports regarding this
property of hairy roots. It is the aim of the present work to
address this particular issue based on the observation of growth and
tropane alkaloid production of one hairy root clone of B. candida
during 5 years of culture.
RESULTS
Once
the B. candinda hairy roots were obtained, one clone (clone
7) was chosen based on its growth rate and scopolamine production
(data not shown). It is important to note that in every clone
examined, tropane alkaloid production and growth were associated (Pitta-Alvarez,
1998). After a few subcultures, the formation of a callus derived
from clone 7 was observed and, consequently, tropane alkaloid
production ceased. The strategies deployed to overcome this
difficulty have been described in Pitta-Alvarez and Giulietti
(1995). It was observed that when the callus was subcultured in B51/2
medium, multiple hairy roots emerged from it. Every root was
considered a different clone and, again, the one with the best
growth rate and scopolamine production (Figures 2a and 2b) was
chosen (clone 7X). In this case, it was evident that the change in
tropane alkaloid production was due to the fact that the system had
undergone a dedifferentiated state with the subsequent genetic
instability derived from this situation. The use of B51/2
medium was used for the remaining subcultures, thus preventing
dedifferentiation (Pitta-Alvarez and Giulietti, 1995). In clone 7X,
alkaloid production and growth were also closely associated (Fig. 2a
and 2b).
During
subculture of clone 7X in B51/2 medium, gradual changes
in tropane alkaloid production and growth were observed. After 3
years, the production of scopolamine had increased significantly,
while the hyoscyamine one remained practically the same as in
previous years (Figure 3a). As a result, the ratio total
scopolamine/total hyoscyamine (St/Ht) increased dramatically.
However, growth was affected negatively (Figure 3b). After five
years of subcultures of the same clone, both scopolamine and
hyoscyamine production were strongly augmented (Figure 3a). The
ratio St/Ht remained practically the same as the one registered
after 3 years of culture. However, growth declined significantly and
finally ceased (Figure 3b).
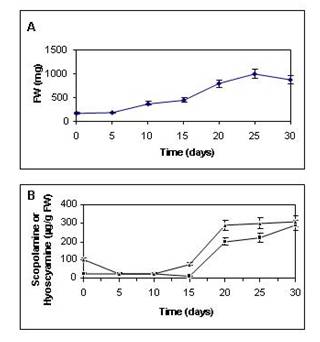
Figure
2.
(a) Kinetics of growth of clone 7X immediately after
re-differentiation with medium B51/2 supplemented with 15 g/l
sucrose. (b) Kinetics of scopolamine and hyoscyamine
production in clone 7X immediately after re-differentiation with
medium B51/2 supplemented with 15 g/l sucrose. FW: Fresh weight. Dry
weight represented in every case approximately 10% of total FW.
--▲--: Scopolamine; --■--: Hyoscyamine.
DISCUSSION
The
exact reasons for the observations described above remain unclear.
Although the functions of tropane alkaloids in plants are not yet
completely elucidated, they apparently are related to defense
against herbivores. Since in vitro cultures represent in
themselves a stressful situation, they could trigger in the cultures
the production of these alkaloids. In addition, there is a
possibility that other metabolites that appear only in in vitro
cultures could be involved in promoting the production of tropane
alkaloids through the years. For instance, cadaverine, which is
absent in the plant, was detected in these hairy roots (Carrizo et
al, 2001). Although this particular polyamine is possibly not
responsible for the increased levels of tropane alkaloids, it
suggests that other novel compounds, not found in plants, might
up-regulate the enzymes involved in the biosynthesis of these
alkaloids (Figure 1).
Furthermore, it has been reported that in whole plants of B.
candida, scopolamine is transferred possibly in larger
proportion than hyoscyamine (El-Dabbas and Evans, 1982) to the
aerial parts. Maldonado-Mendoza et al. (1993) found that hairy roots
of Datura stramonium produced tropane alkaloids 2 orders of
magnitude higher than mother plants. They suggested that this
because in mother plants both metabolites are translocated and
stored in aerial parts of the plant (Payne et al.,1987). The
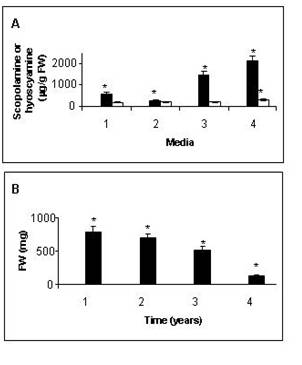
Figure
3. (a)
Modifications of tropane alkaloid production over a 5 year period of
subculture in two different media: 1: MS supplemented with 30 g/l
sucrose employed until dedifferentiation took place; 2,3 and 4:
B51/2 with 15 g/l sucrose employed (2) after re-differentiation, (3)
after 3 years of subculture and (4) after 5 y. of subculture. The
samples were taken after 20-d. of culture. FW: Fresh weight. Each
value represents the mean of three independent determinatiosns. Data
marked with an asterisk are significantly different according to
Tukey´s test (p=0.05). Dry weight represented in every case
approximately 10% of total FW. --■-- Scopolamine; --□—Hyoscyamine.
(b) Modifications of growth over a five-year period of
subculture in two different media: 1: MS supplemented with 30 g/l
sucrose employed until dedifferentiation took place; 2,3 and 4:
B51/2 with 15 g/l sucrose employed (2) after re-differentiation, (3)
after 3 y. of subculture and (4) after 5 y. of subculture. The
samples were taken after 15-d. of culture. FW: Fresh weight. Dry
weight represented in every case approximately 10% of total FW. Each
value represents the mean of three independent determinatiosns. Data
marked with an asterisk are significantly different according to
Tukey´s test (p=0.05).
fact
that this molecule cannot be translocated in root cultures could
induce a larger accumulation. The limitations to this accumulation
could be determined by negative feedback mechanisms or by the
incapacity of the root to tolerate its toxicity. This last
speculation would explain the negative effect on growth observed as
tropane alkaloid accumulation continued to rise in our hairy root
system.
Yukimune et al. (1994) used repeated selection in transformed root
cultures of Duboisia myoporoides. They found that in hairy
roots the scopolamine content of the lines obtained at each
selection increased with the number of selections. They also
observed that the morphology of the hairy roots with improved
scopolamine content differed after the repeated selection,
obtainining fine root lines with extensive lateral branching. The
authors suggested that the initial root tip consisted in
heterogenous cells, even though it had been established from one
root tip. Following that hypothesis, they concluded that the highly
productive root lines obtained from repeated selection must be the
result of the removal of the heterogenous cells. Our roots could
have suffered a similar phenomenon, although we did not observe
morphological differences in the process. In agreement with Yukimune
et al. (1994), the growth rates of clone 7X decreased after repeated
selection. A similar mechanism could be at play in our hairy roots,
although it is intriguing that in our case the selected phenotype
was always the one with increased levels of scopolamine.
There
have been numerous reports of long-term stability in alkaloid
production. Maldonado-Mendoza et al. (1993) reported that, in hairy
root lines of D. stramonium, growth patterns, biomass and
tropane alkaloid production remained constant for more than 5 years.
The difference in species used for these experiments could be partly
responsible for the variations observed with our hairy root clone.
In addition, they tested a variety of A. rhizogenes strains,
but not LBA 9402, which is the strain we used in our experiments. At
the moment we are working also with both A. rhizogenes strain
15834 and LBA9402 to see if the same changes in tropane alkaloids
and growth are observed, or if it depends on the strain used and its
interaction with the plant species. In these experiments, we are
examining several clones obtained by either strain of
Agrobacterium. Our observations thus far are in consonance to
the behavior of clone 7X, with increasing concentrations of
scopolamine and a gradual decrease in growth rate.
Baiza
et al. (1999) observed that all the hairy roots lines of D.
stramonium had a stable production over a period of 6 years. It
has to be noted that the species used was the same as
Maldonado-Mendoza (1993) and, perhaps, the hairy roots derived from
them showed a higher stability than other species. They also found
that there was an inverse relationship between growth and secondary
metabolite production. As stated before, in our hairy roots growth
and production were closely related, and this could be involved in
the differences observed with hairy roots of other species.
Mano
et al. (1986) found that the production of tropane alkaloids was
unstable in both normal and hairy root lines established from
Scopolia japonica. However, the hairy root cultures established
for metabolite production were heterogeneous since they consisted of
pools of hairy roots rather than clones. They traced the unstable
metabolic activity to this initial heterogeneity. Therefore, the
results obtained by this group cannot be compared to ours since our
initial inoculum consisted of one root tip, thus assuring that we
were starting with the same genotype.
Guivrac´h et al. (1999) raised the question as to whether the
stability of transformed root phenotypes was correlated with the
stability of gene expression in hairy root cultures. They considered
that the viability and growth potential of the transformed roots in
long-term cultures had not been fully studied. Consequently, they
established hairy root clones from single root tips after the
inoculation of carrot discs with a co-integrated A. rhizogenes
comprising the wild pRi A4 T-DNA and the gus gene. The gus
gene was used as a marker for the presence of TL-DNA and the aux2
and opine synthesis genes served as indicators for the presence of
TR-DNA. They then followed the evolution of the phenotype
characteristics and gene expression following successive subcultures
over a 2-period year. From their results, they concluded that the
observed differences between clones were not correlated with the
transformation events. In addition, all the clones obtained were
capable of growth on hormone-free medium. Furthermore, they observed
large individual variations in growth patterns between clones and,
most importantly from our point of view, also inside single clones
during various sub-cultures. They concluded that both phenotypes and
gene expression in hairy root clones are not completely predictable.
Also, the pRi T-DNA genes may be expressed unstably and gene
silencing must be considered. This is closer to our observations,
but in our case, the variation was always the same: gradual decrease
in growth with a concomitant increase in alkaloid production. The
phenotype was not subjected to any changes. There is a possibility
that our particular clone was not susceptible to possible negative
feedback from scopolamine. As a result, the increased amounts of
scopolamine could be correlated with the up-regulation of H6H, and
this, in contrast to the statements made by Guivrac´h et al. (1999)
may be the consequence of transformation events.
Aird
et al. (1988) suggested that hairy root cultures of several species
have a stable secondary metabolite production as a consequence of
their genetic stability at the chromosomal level. In this respect,
Baíza et al. (1999) studied the kariotypic stability of 3 lines of
hairy roots, with stable production, of D. stramonium versus
instability of non-transformed roots of the same species. They found
that the transformed cultures consisted, cytologically, exclusively
of diploid cells, while non-transformed ones presented mixoploidy
and aneusometry. The kariotype of the hairy root cultures were the
same as that of the plant root tips, and this stability remained
irrespective of the age of transformed cultures. Transformed root
cultures had a stable production of hyoscyamine and scopolamine,
while the normal roots showed a marked instability through time. It
has been proposed that normal roots are unstable because auxins
inhibit the synthesis of alkaloids through the inhibition of PMT
(Wagner et al., 1986) while others consider that auxins that are
applied exogenously induce chromosome alterations (Nagl, 1986;
Murata, 1989). The roots obtained by our group were apparently
particularly susceptible to auxins (Pitta-Alvarez and Giulietti,
1995), and this could constitute an explanation for their
instability. Even though the problem of dedifferentiation was solved
(Pitta-Alvarez and Giulietti, 1995) the growth pattern of the
selected clone was not stable and there was a definite increase in
scopolamine production. This could be explained by an apparent
random event in the transformation process or to different responses
to auxins.
Jouhikainene et al. (1999), who worked with hairy roots of
Hyoscyamus muticus overexpressing the gene for H6H to enhance
the production of scopolamine, reported observing considerable
variation between the clones both in morphology as well as
production of hyoscyamine and scopolamine. This could have been the
result of using different A. rhizogenes strains, but the
difference was also seen within groups. Nonetheless, they also
reported that scopolamine production of one of these transgenic
hairy roots remained stable during 2 and a half years of
cultivation.
In
contrast to the results obtained by our group, Dechaux and Boitel-Conti
(2005), working with hairy roots of Datura innoxia that
overexpressed H6H from H. niger, observed a decrease in
scopolamine levels similar to control levels after one-year of
subcultures, while hyoscyamine amounts remained the same. They also
studied the transcription level of the h6h gene and found
that it did not decrease after two years of culture. As a result,
they concluded that the decrease of scopolamine accumulation was not
due to molecular modification of the exogenous h6h gene.
Although growth of the hairy roots was diminished with respect to
the control, they did not find a direct relation between growth
ability and scopolamine production, and they concluded that, despite
the toxicity of this molecule, the lower growth could be due to
insufficient medium supplementation for the lines´ growth
requirements. However, as the hairy roots of D. inoxia were
not able to stably overaccumulate scopolamine, they proposed that
scopolamine content may be subjected to metabolic regulation. They
speculated that the high content of scopolamine accumulated in hairy
roots, which have no storage structure or function, seems to be an
enzymatic regulation signal. On the other hand, Palazón et al
(2003), who obtained Duboisia lines overproducers of
scopolamine, observed that adding H6H activity in this plant led to
a better conversion of hyoscyamine to scopolamine. They consequently
concluded that hyoscyamine may be a feedback regulation signal. In
the transformed lines they obtained, the inhibition was removed by
the production of scopolamine. However, they suggested that each
genus seems to possess its own regulation pathway
It is
important to highlight the fact that the hairy roots obtained in our
laboratory were not engineered to overexpress H6H, and therefore the
changes observed cannot be attributed to higher amounts of this
enzyme. A distinct possibility could be that due to the stress of
in vitro culture, the whole biosynthetic pathway could be
up-regulated, in particular the bioconversion of hyoscyamine into
scopolamine. In addition, this particular metabolic pathway is
highly regulated. If in normal roots scopolamine was the main
regulator through negative feedback mechanisms, it could be
hypothesized that in hairy roots of B. candida this mechanism
could be lost. Since this is a highly regulated pathway, there could
have been a de-regulation, with scopolamine losing the ability to
negative feedback.
CONCLUSIONS
Since
its inception, the genetic and phenotypic stability of transformed
roots with A. rhizogenes has been one of the pillars in their
preferential use over suspension cultures. However, and as this
paper demonstrates, the reports pertaining to this particular
characteristic are contradictory. In our case, stability was lost
over a 5 year period of subculture, and significant increases in
scopolamine production as opposed to diminished growth could be
observed. De-regulation of certain genes that are key in the
biosynthetic pathway could be involved in the results observed.
However, further studies, particularly at the molecular level and
with a higher number of clones, have to be initiated to determine if
subculture of the roots, and the consequent stress applied, affect
their stability or if some other mechanism such as genetic
instability could be playing a role. Nevertheless, from the cited
data, it is clear that stability must be permanently controlled,
particularly if long-term industrial processes are entailed.
MATERIALS AND METHODS
Establishment and maintenance of hairy root cultures
Transformed (hairy) root cultures were obtained by infecting
explants of B. candida with Agrobacterium rhizogenes
LBA 9402, employing the procedure described in Pitta-Alvarez and
Giulietti (1995). The establishment of the cultures and the
confirmation of their transformation were carried out according to
Pitta-Alvarez and Giulietti (1995). The roots were maintained first
in liquid Murashige and Skoog medium (MS) (1962) supplemented with
30 g/l sucrose. The medium used for subsequent subcultures was
Gamborg (Gamborg et al., 1968) liquid medium with half-concentration
of mineral salts and vitamins (B51/2) supplemented with
15 g/l sucrose. The roots were subcultured in the media described
every 20 days and incubated at 24±2°C, in gyratory shakers at 100
rpm with a 16-h photoperiod by using cool white fluorescent lamps at
a light intensity of approximately 1.8 W/m2. This procedure was
followed for a period of 5 years.
Analytical methods
Fresh
weight (FW) was determined by separating the root tissue from the
medium by vacuum filtration. Alkaloid extraction was carried out as
described by Parr et al. (1990). This consisted in the treatment of
the hairy roots with 0.2% sulfuric acid during two hours. After
washing the roots, they were exposed to NaOH 1N and the alkaloids
were removed with CHCl3. The chloroform phase was
evaporated and the residue was used to determine tropane alkaloids.
Hyoscyamine and scopolamine were analyzed by high-performance liquid
chromatography, according to the method described by Mano et al.
(1986).The determinations were carried out in a Kontron HPLC with a
Kontron spectrophotometer UV 430. The column used was a Spherisorb
S5 ODS2 250 X 4.6 mm. The mobile phase was constituted by 1%
triethylamine:ethanol (9:1) (pH = 3.5 with formic acid). The
absorption was read at a wavelength of 230 nm. Scopolamine had a
retention time of 7 min., and hyoscyamine of 15 min. Dry weight (DW)
was determined by drying the roots at a temperature of 100°C until
constant weight.
Chemicals
Scopolamine, (-)-hyoscyamine, and all the media components were
purchased from Sigma Chemical Co. (St. Louis, MO, USA)
Statistical analysis
Significance of treatments was determined by using analysis of
variance. Variations among the means of the treatment were analyzed
by Tukey´s procedure (1953) (p=0.05).
LITERATURE CITED
Aird
ELH, Hamill JD, Rhodes MJC
(1988) Cytogenetic analysis of hairy root cultures from a number of
plant species transformed by Agrobacterium rhizogenes. Plant
Cell Tiss Org Cult 15:47-57.
Baíza
AM, Quiroz A, Ruia JA, Maldonado-Mendoza I, Loyola-Vargas VM
(1998) Growth patterns and alkaloid accumulation in hairy root and
untransformed root culture of Datura stramonium. Plant Cell
Tiss and Org Cult 54:123-130.
Baíza
AM, Quiroz-Moreno A, Ruiz JA, Loyola-Vargas VM
(1999) Genetic stability of hairy root cultures of Datura
stramonium. Plant Cell, Tiss and Org Culture. 59:9-17.
Carrizo CN, Pitta-Alvarez SI, Kogan MJ, Giulietti AM, Tomaro ML
(2001) Occurrence of cadaverine in hairy roots of Brugmansia
candida. Phytochemistry 57:759-763.
Chilton JD, Tepfer DA, Petit A, David C, Casse-Delbert, J, Tempe J
(1982) Agrobacterium rhizogenes inserts T-DNA into the genome
of the host plant root cells. Nature 295:432-434.
Chilton MD, Drummond MH, Merio DJ, Sciaky D, Montoya AL, Gordon MP,
Nester EW
(1977) Stable incorporation of plasmid DNA into higher plant cells:
the molecular basis of crown gall tumorigenesis. Cell 1:263-271.
Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AM
(2006) Recent developments and future prospects in insect pest
control in transgenic crops. Trends Plant Sci, 11:302-308.
Dechaux C, Boite-Conti M
(2005) A stategy for overaccumulation of scopolamine in Datura
innoxia hairy root cultures. Acta Biologica Cracoviensia:Series
Botanica 47:101-107.
Flores
HE, Hoy MW, Pickard JJ
(1987) Secondary metabolites from root cultures. Trends Biotechnol
5:64-69.
Gamborg OL, Miller RA, Ojima K
(1968) Nutrient requirements of suspension cultures of soybean root
cells. Exp Cell Res 50:151-158.
Glaser
JA, Matten SR
(2003) Sustainability of insect resistance management strategies for
transgenic Bt corn. Biotechnology Advances 22:45-69.
Guivarc´h A, Boccara M, Proteau M, Chriqui D
(1999) Instability of phenotype and gene expression in long-term
cultures of carrot hairy root clones. Plant Cell Rep 19:43-50.
Hamill
JD, Parr AJ, Robins RJ, Rhodes MJC
(1986) Secondary product formation by cultures of Beta vulgaris
and Nicotiana rustica transformed with Agrobacterium
rhizogenes. Plant Cell Rep 5:111-114.
Hashimoto T, Hayashi A, Amon A, Kohno J, Iwanari H, Usuda S, Yamada
Y
(1991) Hyoscyamine-6-β-hydroxylase, an enzyme involved in tropane
alkaloid biosynthesis, is localized at the pericycle of the root. J
Biol Chem 266:4648-4653.
Hashimoto T, Yun D-J, Yamada Y
(1993) Production of tropane alkaloids in genetically engineered
root cultures. Phytochemistry 32:713-718.
Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teeri HT,
Oksman-Caldentey K-M
(1999) Enhancement of scopolamine production in Hyoscyamus
muticus L. hairy root cultures by genetic engineering. Planta
208:545-551.
Kamada
H, Okamura N, Satake M, Harada H, Shimomura K
(1986) Alkaloid production by hairy roots cultures in Atropa
belladonna. Plant Cell Rep 5:239-242.
Maldonado-Mendoza IE, Ayora-Talavera T, Loyola-Vargas VM
(1993) Establisment of hairy root cultures of Datura stramonium.
Characterization and stability of tropane alkaloid production during
long periods of sub-culturing. Plant Cell Tiss and Org Cult
33:321-329.
Mano
Y, Nabeshima S, Matsui C, Ohkawa H
(1986) Production of tropane alkloids by hairy root cultures of
Scopolia japonica. Agri Biol Chem 50:2715-2722.
Matsuda J, Okabe S, Hashimoto T, Yamada Y
(1991) Molecular cloning of hyoscyamine-6-β-hydroxylase, a
2-oxoglutarate dioxygenase, from cultured roots of Hyoscyamus niger.
J Biol Chem 266:9460-9464.
Murashige T , Skoog F
(1962) A revised medium for rapid growth and bioassays with tobacco
tissue cultures. Physiol Plant 15: 473-497.
Murata
M
(1989) Effects of auxin and cytokinin on induction of sister
chromatid exchanges in cultured cells of wheat (Triticum aestivum
L.) Theor Appl Genet 78:521-524.
Nagl W
(1986) Genome changes induced by auxin-herbicides in seedlings and
calli of Zea mays L. Environ Exp Bot 28:197-206.
Palazon J, Moyano E, Cusido RM, Bonfill M, Oksman Caldentey KM,
Pinol MT
(2003) Alkaloid production in Duboisia hybrid hairy roots and plant
overexpressing the h6h gene. Plant Sci 165:1289-1295.
Parr
AJ, Payne J, Eagle J, Chapman B, Robins RJ, Rhodes MJC
(1990) Variation in tropane alkaloid accumulation within the
Solanaceae and strategies for its exploitation. Phytochemistry
29:2545-2550.
Payne
J, Hamill JD, Robins RJ, Rhodes MJC
(1987) Production of hyoscyamine by hairy root cultures of Datura
stramonium. Planta Med 53:474-478.
Pitta-Alvarez
SI, Giulietti AM
(1995) Advantages and limitations in the use of hairy root cultures
for the production of tropane alkaloids: use of anti-auxins in the
maintenance of normal root morphology. In Vitro Cellular and
Developmental Biology-Plant. 31:215-220.
Pitta-Alvarez
SI
(1998) In vitro production of tropane alkaloids employing
transformed roots of Brugmansia candida. PhD thesis.
University of Buenos Aires, Buenos Aires, Argentina.
Tukey
JW
(1953) Some selected quick and easy methods of statistical analysis.
Trans NY Acad Sci Ser II 16:88-97.
Wagner
R, Feth F, Wagner KG
(1986) The regulation of enzyme activities of the nicotine pathway
in tobacco. Physiol Plant 68:667-672.
Wisniewski J-P, Frangne N, Massonneau A, Dumas C
(2002) Between myth and reality: genetically modified maize, an
example of a sizeable scientific controversy. Biochimie
84:1095-1103.
Yukimune Y, Hara Y, Yamada Y
(1994) Tropane alkaloid production in root cultures of Duboisia
myoporoides obtained by repeated selection. Biosci Biotech
Biochem 58:1443-1446.
Accepted for
publication: 15 October 2008
|