ABSTRACT
Environmental pollution is a global concern that is
threatening the well-being of all life forms including
humans. The cost of cleaning up contaminated sites is high
and phytoremediation, the use of plants for removal of
environmental pollutants, offers an attractive option due to
its low cost and safety of implementation. The hairy roots
technology has potential to become an excellent platform for
studying numerous aspects encompassing phytoremediation.
This is because hairy roots can be grown in large mass in
culture media in a controlled environment and can therefore
be subjected to various physiological assays. Also, these
transformed roots are amenable to genetic manipulation and
may facilitate the characterization of genes that influence
the phytoremediation capacity of plants. This idea is well
supported by the recent success in the development of
transgenic plants for use in phytoremediation. Thus, hairy
roots offer a good opportunity for the initial assessment of
transgene efficacy in phytoremediation. Also, in the near
future, hairy roots might be developed into initial screens
for plants with enhanced capacity for phytoremediation. This
review highlights the recent advances in the use of hairy
roots to assess plants for their
potential in removing important water and soil pollutants
such as metals, explosives, radionuclides, insecticides, and
antibiotics.
Environmental pollution is a global
concern
Environmental pollution is a
global problem that affects both the developing and
developed countries (Suresh and Ravishankar, 2004). To a
large extent, both human and natural processes contribute to
environmental pollution and contaminants are commonly
classified as either organic or inorganic. Organic
contaminants are a result of human activities including oil
spills, military explosives, agriculture, fuel production,
and wood treatment (Pilon-Smits, 2005). Common organic
pollutants such as trichloroethylene (TCE), herbicides such
as atrazine, explosives such as trinitrotoluene,
petrochemicals such as benzene, toluene, polycyclic aromatic
hydrocarbons, polychlorinated biphenyls (PCBs), and the fuel
additive methyl tert-butyl ether may contaminate
soils and water (Xingmao and Burken, 2003; Pilon-Smits,
2005; Rentz et al., 2005; Suresh et al., 2005; González et
al., 2006). In general, inorganic contaminants originate
from either natural processes of soil weathering or human
activities including agriculture and mining (Pilon-Smits,
2005). Subsequently, both natural and human activities may
promote the release of heavy metals e.g. manganese, lead,
copper, zinc, molybdenum, mercury, and nickel into soils and
water posing a health threat to livestock and human
populations (Nedelkoska and Doran, 2000a). For example,
mercury is an important health concern to populations that
rely heavily on the consumption of fish as a protein source
(Hajeb et al., 2008 ), and to a large extent all global
water bodies face the threat of mercury contamination
(Harris et al., 2007).
Plants are used to remove
environmental contaminants
The health consequences
due to environmental pollution are dire and the cost of
cleaning up contaminated sites is high (Kuiper et al., 2004;
Doty, 2008). Therefore, the use of plants to absorb,
stabilize and degrade contaminants, collectively referred to
as phytoremediation, is gaining acceptance as a more
cost-effective alternative to other cleanup approaches.
Phytoremediation is a technology that has been extensively
reviewed (for recent reviews see Suresh and Ravishankar,
2004; Pilon-Smits 2005, and Doty, 2008). Our intention here
is not to duplicate the efforts of the experts in the field,
but instead we will concentrate this review on the potential
of hairy roots as a powerful tool to study the
phytoremediation capacity of plants.
The process of contaminant extraction by plants and the
subsequent fates of the contaminant are described in Figure
1. Plant roots may act as a conduit for the absorption of a
contaminant which is then translocated through the vascular
system and concentrated in plant harvestable tissues in a
process called phytoextraction (Doty, 2008). In addition,
roots may provide a haven for microbial growth by secreting
exudates that in turn act as a source of nutrition for the
microbes and also serve as important cues for enhancing
plant-microbe interactions (Bais et al., 2006). The
resulting rhizospheric interactions may enhance the
biodegradation of organic contaminants in a process referred
to as phytostimulation (Pilon-Smits, 2005 and references
therein). Prior and after entering the plant via the root
system, the contaminant may become target for degradation by
either secreted or internal plant enzymes in a process
called phytodegradation (Boominathan et al., 2004; Doty,
2008). The phytoremediation of some organic contaminants
(e.g. TCE) is influenced by its concentration and the rate
of transpiration, and TCE may be released from the plant
through volatilization (Xingmao and Burken, 2003). Thus, in
phytoremediation plants are used to facilitate optimum
conditions for microbial break down of contaminants and to
extract contaminants which may be metabolized or sequestered
inside the plant (Boominathan et al., 2004; Tamaoki et al.,
2005). Even though the rate of detoxification of organic
contaminants in plant tissue is slow (Van Aken, 2008), the
rising costs of physicochemical cleanup methods of
contaminated sites makes phytoremediation a more attractive
alternative (Doty, 2008 and references therein).
In order to mitigate the downward-migration of contaminants
to the below-ground water reservoirs and lateral movement of
contaminants via runoff and wind erosion, fast-transpiring
trees e.g. poplar (Populus sp.) are grown together
with grasses resulting in phytostabilization of contaminants
(Pilon-Smits, 2005). Therefore, in phytoremediation, plants
provide dual benefits; they play the role of providing
optimum conditions for root colonizing bacteria and also
provide a simple and cost-effective way of extracting
contaminants (Suresh and Ravishankar, 2004). Since roots are
the primary contact between plant tissues and contaminants
in the soil or water they provide a key point for assessment
of the phytoremediation potential of a particular plant
species. The underground portion of a plant system where
roots are in contact with the micro biota is referred to as
the rhizosphere (Walker et al., 2003) and the interaction
among plant, microbes and mycorrhizal colonies is regulated
to a large extent by root exudates (Walker et al., 2003;
Bais et al., 2006). To that regard, root exudates are an
essential component for pollutant degradation by microbes in
the rhizosphere, and rhizosphere processes are thought to be
essential for facilitating the uptake of contaminants by
plants (Rentz et al., 2005). Therefore, the root environment
and interactions among roots and microorganisms are key
aspects to consider in phytoremediation (Barea et al.,
2005).
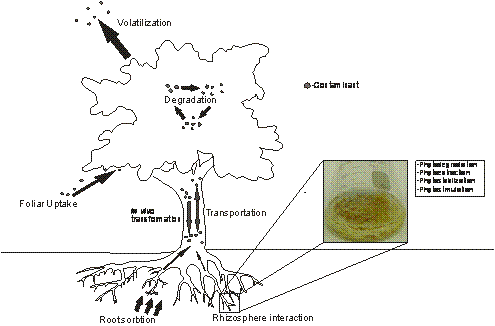
Figure 1.
Uptake and metabolism of environmental contaminants by
plants: Contaminants can be absorbed by roots and foliage,
transformed and degraded in planta, or volatilized
into the atmosphere; rhizosphere interactions may also
contribute to extraction and degradation of contaminants
during phytoremediation. Hairy roots are a powerful tool to
study various key processes that impact the overall
phytoremediation capacity of plants, i.e. the rate of
pollutant degradation, extraction, or stabilization. Hairy
roots can also be used to study how root exudates may
stimulate the degradation of particular contaminants.
Hairy roots biotechnology for valuable
metabolite production
Hairy roots are fine fibrous structures that are formed on
plant tissues infected by Agrobacterium rhizogenes, a
soil bacterium responsible for the root mat disease (Georgiev
et al., 2007; Veena and Taylor, 2007). After infecting the
cells, A. rhizogenes stably transfers several of its
genes to the plant genome resulting in physiologic changes
in the host cell leading to enhanced growth in hormone-free
media (Srivastava and Srivastava, 2007). The observed
changes in root physiology and morphology are associated
with the transfer of a cluster of genes from the A.
rhizogenes large Ri (root-inducing) plasmid into the
plant genome. The symptoms observed with A. rhizogenes
infection may suggest that the transformed cells have been
rendered more sensitive to auxin without altering the
production of these plant hormones (McAfee et al., 1993;
Srivastava and Srivastava, 2007).
Humankind has tapped into
the plant natural products reservoir not only for
nutritional needs, but also for medicinal and aesthetic
purposes (Srivastava and Srivastava, 2007). However, to a
high degree most valuable plant natural products are
produced in small amounts from specialized metabolic
pathways that fluctuate with respect to environmental
conditions. The versatility of the hairy roots system has
allowed the development of platforms for the production of
high-value natural products, at times in scaled up
bioreactors (Georgiev et al., 2007; Cuello and Yue, 2008;
Villarreal et al., 2008; Weathers et al., 2008). In
addition, the inherent characteristics of hairy roots
including their fast growth, genetic stability, short
doubling time, and ability to produce a broad range of
metabolites similar to wild type make this system a powerful
tool for metabolic engineering (Veena and Taylor, 2007). In
combination with transgenic approaches, the capacity of
hairy roots metabolism can be manipulated for the
enhancement of de novo synthesis of high value
phytochemicals (Guillon et al., 2006).
Hairy roots technology offers important advantages for
phytoremediation studies
Hairy roots offer several
advantages for use in phytoremediation studies, these
include: their ability to grow rapidly in microbe-free
conditions, providing a greater surface area of contact
between contaminant and tissue, and they are genetically and
metabolically more stable in comparison to wild type (Gujarathi
et al., 2005; Georgiev et al., 2007). Hairy roots are also
amenable to genetic transformation, making gene transfer and
characterization possible in a system that may pose minimum
health or environmental concerns. Another advantage of using
hairy roots for studying phytoremediation is their ability
to produce large quantities of exudates which are composed
of enzymes and some metal chelating compounds that may
detoxify or sequester harmful organic and inorganic
contaminants (Gujarathi et al., 2005; Bais et al., 2006;
Doty, 2008). As shown in Table 1, hairy roots have been used
to assess the potential of several plant species to remove
contaminants from the environment. For example, the hairy
root cultures of black nightshade (Solanum nigrum)
may metabolize and remove PCBs from solutions spiked with
PCB congeners (Macková et al., 1997a,b; Kučerova et al.,
2000; Rezek et al., 2007). Also, by studying the rates of
removal and the fate of contaminants such as the explosives
hexahydro-1,3-5-trinitro-1,3-5-triazine (RDX) and
octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX),
Badhra et al. (2001) discovered that periwinkle (Catharanthus
roseus) hairy roots have an “intrinsic ability” to
remove these molecules from the medium. RDX and HMX are the
two most common pollutants found in military sites where
explosives are commonly tested (Pilon-Smits, 2005).
Recently, hairy roots have
been used to test plants for their ability to tolerate high
levels of phenols (de Araujo et al., 2002). Phenols are
commonly used in various agricultural applications or
released from coal and petroleum refining activities, and
they pose a threat to human health (de Araujo et al., 2002;
Agostini et al., 2003; Coniglio et al., 2008). In hairy
roots of carrot (Daucus carota) and other plant
species the role of peroxidase enzymes might be the key
factor in the removal of phenol and chlorophenols from the
culture medium (Agostini et al., 2003; González et al.,
2006; de Araujo et al., 2006; Singh et al., 2006; Coniglio
et al., 2008). Also, the inherent activity of peroxidases in
hairy roots of rapeseed (Brassica napus) was
associated with the effective removal of 2,4-dichlorophenol
and phenol from the medium for several cycles and the
removal process was enhanced by exogenously-applied hydrogen
peroxide (Agostini et al., 2003; Coniglio et al., 2008). It
appears that other plants use additional mechanisms to
remove phenol. For instance, cells of carrot, kangaroo apple
(Solanum aviculare) and sweet potato (Ipomoea
batatas) hairy roots are able to incorporate and
conjugate phenolic compounds with polar cellular materials
(possibly sugars and proteins) as well as with insoluble
materials such as cell walls and membranes (de Araujo et
al., 2006).
To a greater extent, the
ability of plants to metabolize contaminants will depend on
the biochemical characteristics of metabolizing enzymes and
other protective mechanisms that may prolong tissue
survival. Indeed, results from a comparative study of
peroxidase enzymes from hairy roots of carrots, sweet potato
and kangaroo apple demonstrated an inter-specific variation
in the preference for phenol and chlorophenol among
peroxidases (de Araujo et al., 2004). Also, peroxidase
isozymes involved in phenol removal within a species may
show variation in substrate preference and catalytic
efficiency of phenol metabolism (Coniglio et al., 2008). It
is noteworthy that, these studies are important in
establishing an understanding of the enzymatic mechanisms of
contaminant degradation for the selection of candidate
enzymes that might be produced in large amounts and used as
catalysts for contaminant break down (González et al.,
2006).
An inspiring study by
Eapen et al. (2003) demonstrated that hairy roots of the
Indian mustard (B. juncea) and Chenopodium
amaranticolor could remove uranium from solutions and
could withstand high concentrations of this radionuclide for
days. It is encouraging to imagine that in the near future
it may become possible to use plants to cleanup sites
contaminated with radioactive waste and alleviate the
devastating environmental problems that may arise through
uranium contamination of soils and water (Gavrilkescu et
al., 2008).
The uptake of metals and
their distribution in plant tissues are both important
aspects governing the capacity of plants to remove heavy
metals from the soil. Hairy roots have demonstrated that
they can be used as a means for screening a wide variety of
plant species for their capacity to extract and sequester
metals (Nedelkoska and Doran, 2000a). A comparative
assessment of nickel tolerance between hairy roots and whole
plants revealed that the translocation of nickel to above
ground shoots may not be required for nickel tolerance and
hyperaccumulation in certain species of Alyssum (Nedelkoska
and Doran, 2001). This suggests that nickel tolerance may be
conferred by a reduced oxidative damage of hairy roots
tissue due to enhanced catalase activity (Boominathan and
Doran, 2002). Therefore, additional mechanisms to metal
translocation and accumulation in shoots of
hyperaccumulators may play a significant role in heavy metal
tolerance. Indeed, using hairy roots, Boominathan and Doran
(2003a) demonstrated that cadmium was extracted by alpine
pennygrass (Thlaspi caerulescens) and accumulated in
high levels in complexes with organic acids inside the cell
walls.
Table 1.
Phytoremediation of
various environmental pollutants by hairy root cultures as
tools to study the uptake and degradation of xenobiotics
|
Plant species |
Model pollutant |
Reference |
|
Black nightshade (Solanum
nigrum)
Alpine pennygrass
(Thlaspi caerulescens) |
PCBs
Cadmium |
Macková et al.
(1997a; b)
Nedelkoska and
Doran (2000b) |
|
Alyssum
sp. |
Nickel |
Nedelkoska and
Doran (2001) |
|
Periwinkle (Catharanthus
roseus) |
RDX and HMX |
Bhadra et al.
(2001) |
|
Carrot (Daucus
carota) |
Phenol and
chloroderivatives |
de Araujo et al.
(2002) |
|
Wild mustard (Alyssum
bertolonii) and
alpine pennygrass
(T. caerulescens) |
Nickel, and
cadmium
|
Boominathan and
Doran (2002)
|
|
Deadly nightshade
(Atropa belladonna) |
TCE |
Banerjee et al.
(2002) |
|
Rapeseed (Brassica napus) |
2,4-Dichlorophenol |
Agostini et al.
(2003) |
|
Indian mustard (Brassica
juncea) and Chenopodium amaranticolor |
Uranium |
Eapen et al.
(2003)
|
|
Indian mustard (B.
juncea) and chicory (Cichorium intybus) |
DDT |
Suresh et al.
(2005) |
|
Sunflower (Helianthus
annuus) |
Tetracycline and
oxytetracycline |
Gujarathi et al.
(2005) |
|
Tomato (Lycopersicon
esculentum) |
Phenols |
Oller et al.
(2005) |
|
Carrot (D.
carota), sweet potato (Ipomoea batatas),
and kangaroo apple (Solanum aviculare) |
Guaiacol,
catechol, phenol, 2-chlorophenol, and
2,6-dichlorophenol |
de Araujo et al.
(2004; 2006)
|
|
Indian mustard (B.
juncea) |
Phenol |
Singh et al.
(2006) |
|
Tomato (L.
esculentum) |
Phenol |
Wevar-Oller et al.
(2005); González et al. (2006) |
|
Rapeseed (B. napus) |
Phenol |
Coniglio et al.
(2008) |
|
Yellow tuft (Alyssum murale) |
Nickel |
Vinterhalter et
al. (2008) |
|
|
|
|
DDT= Dichloro-diphenyl-trichloroethane;,
HMX=oxtahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine; PCBs =
polychlorinated biphenyls; RDX=hexahydro-1,3-5-trinitro-1,3-5-triazine;
TCE=Trichloroethylene;
Also, in another study,
Boominathan and Doran (2003b) revealed that an inherent high
catalase activity may play an important role in cadmium
hyperaccumulation in T. caerulescens hairy roots.
Therefore, the establishment of hairy root cultures for a
variety of plant species might be a good strategy in studies
of growth and heavy metal tolerance in plants (Nedelkoska
and Doran, 2000b). Ultimately, the application of tissue
culture technology may prove powerful in the regeneration of
shoot cultures from hairy roots of selected species of
plants with superior phytoremediation traits (Vinterhalter
et al., 2008).
It is important to monitor
and limit the release of pesticides and antibiotics into the
environment, and of equal importance is the identification
of methods for cleanup in the case of contamination. Hairy
roots of sunflower (Helianthus annuus) are
effective in extracting and metabolizing antibiotics
including tetracycline and oxytetracycline through a process
that is thought to involve reactive oxygen intermediates (Gujarathi
and Linden, 2005). There is controversy regarding the
continuous use of the insecticide DDT to combat mosquitoes
that spread malaria in developing countries (Sadasivaiah et
al., 2007) even though some studies suggest that DDT might
have negative health effects on human health (Hatcher et
al., 2008). Hairy roots of chicory (Cichorium intybus)
and Indian mustard (Brassica juncea) have been
used to study their potential in removing DDT from
contaminated sites (Suresh et al., 2005). Interestingly,
C. intybus and B. juncea might produce enzymes
that degrade DDT (Suresh et al., 2005), thus offering a
promising possibility for the characterization of these
enzyme(s) and for similar studies to be done in other plant
species.
The expression of
heterologous proteins in hairy roots has successfully been
done (Banerjee et al., 2002). Such an approach was used to
express a mammalian cytochrome P450 enzyme in deadly
nightshade (Atropa belladonna) and the transgenic
plants were able to metabolize the environmental pollutant
TCE (Bernejee et al., 2002). Five years later, Doty et al.
(2007) were successful in transforming poplar (Populus
tremula x Populus alba) with this mammalian
enzyme to generate plants with a superior capacity to remove
various organic pollutants from hydroponic solutions and
air. Of the several lines transformed with the mammalian
enzyme, line 78 metabolized TCE a hundred-fold more than
non-transgenic control trees (Doty et al., 2007). Also,
others have used transgenic approaches that involved the
over-expression of plant genes encoding contaminant
metabolizing enzymes in hairy roots. For example, by
over-expressing a tomato (Lycopersicon esculentum)
tpx1 gene encoding a peroxidase in hairy roots,
Wevar-Oller et al. (2005) generated roots with enhanced
capacity of removing phenol from the medium. These studies
demonstrated that transgenic approaches may be adopted to
produce plants with novel and improved phytoremediation
capacity (Van Aken, 2008). Therefore, in the near future the
use of transgenic hairy root systems may become more common
in testing the efficacy of transgenes and the enzymes they
encode for the removal of hazardous environmental
pollutants.
All these studies
demonstrate the power of using hairy roots in screening for
candidate genes involved in the metabolism of environmental
contaminants. Figure 2 illustrates a model of the
mechanism(s) by which wild type or transgenic hairy root
cells may metabolize environmental contaminants. It is
noteworthy, however, that although the generation of
transgenic plants with enhanced phytoremediation capacity
might seem as a plausible solution, public skepticism and
resistance to transgenic organisms might make this option
less favorable for application in the near future.
Alternatively, the selection of local plant species with
enhanced phytoremediation capacity through hairy root
screens may become more favorable and practical in the
immediate future.
CONCLUSIONS AND FUTURE DIRECTIONS
Hairy roots can be
generated from many plant species by infecting them with
A. rhizogenes. This technology has facilitated a more
stable production of important medicinal and high-value
products at times in scaled up bioreactors. The versatility
of hairy roots makes this system more attractive for the
assessment of various physiological aspects of plants. The
problem of environmental pollution affects both local and
global human populations and physicochemical technologies of
environmental cleanup are costly. Therefore, the use of
plants in phytoremediation is gaining more support. Plants
have intrinsic abilities to extract and metabolize
contaminants and their cooperation with soil microorganisms
and endophytes, microbes that live inside plants, may
enhance the removal of contaminants from the environment.
However, it is conceivable that not all species will possess
superior capacities to extract and metabolize pollutants.
These valuable plant traits can be screened for using hairy
root cultures. Thus, the initial selection of superior plant
species for use in phytoremediation can begin in the
laboratory followed by the actual growing and testing plants
in the greenhouse and the field. As hairy roots are amenable
to genetic transformation, transgenic approaches may be used
to study candidate genes that affect pollutant removal.
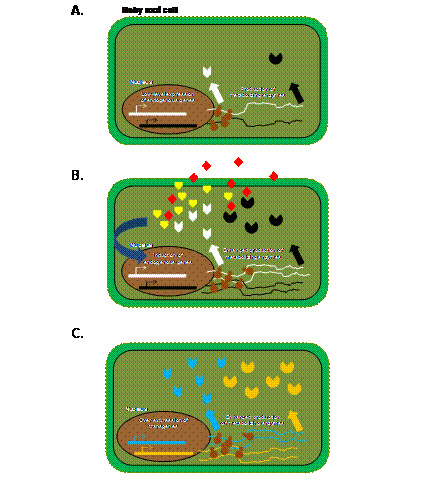
Figure 2.
Metabolism of environmental contaminants by hairy root
cells: (A) a cartoon depiction of a hairy root cell
expressing contaminant metabolizing enzymes (white chevron
and black pie) at basal levels; (B) environmental
contaminants (red diamonds) may promote the production of
reactive oxygen species (yellow pentagon), the enhanced
production of ROS scavenging enzymes and antioxidants (white
chevron), and/or contaminant metabolizing enzymes (black
pie); (C) the expression of transgenes of animal or
plant origin may also result in the enhanced production of
contaminant metabolizing enzymes (blue chevron and orange
pie) and phytoremediation capacity of plants.
Therefore, in the near
future the hairy roots technology might be used more
commonly in biotechnological efforts ranging from metabolite
production to phytoremediation. Despite the large potential
of hairy roots in phytoremediation studies, the ongoing
challenge will be the actual translation of laboratory
results to field applications. The lack of microbes in
axenic hairy roots media may prevent our full appreciation
of the benefits of the rhizospheric organisms that often
enhance the uptake and breakdown of pollutants.
Nevertheless, it is encouraging to witness the recent
development of transgenic plants, poplar trees in
particular, that promise to offer a tremendous impact on
phytoremediation. In summary, hairy roots provide a
promising tool in the field of phytoremediation but the work
of environmental remediation has just begun.
ACKNOWLEDGMENTS
Phytoremediation-related
research at the Lorence Laboratory is funded by the Arkansas
Biosciences Institute, the major research component of the
Arkansas Tobacco Settlement Proceeds Act, and a sub-award
(to AL) from the Arkansas IDeA Network of Biomedical
Research Excellence (NIH-NCCR 5 P20 RR016460-05 to L
Cornett). The authors thank C Ńopo and F Medina-Bolivar for
providing the hairy root picture included in Figure 1.
REFERENCES CITED
Agostini E, Coniglio MS,
Milrad SR, Tigier HA, Giulietti AM (2003) Phytoremediation of
2,4-dichlorophenol by Brassica napus hairy root cultures.
Biotechnol Appl Biochem 37: 139-144
Bais HP, Weir TL, Perry
LG, Gilroy S, Vivanco JM (2006) The role of root exudates in
rhizosphere interactions with plants and other organisms. Annu
Rev Plant Biol 57: 233-266
Barea J, Pozo JM, Azcón R,
Azcón-Aguilar C (2005) Microbial cooperation in the rhizosphere.
J Expt Bot 56: 1761-1778
Bernejee S, Shang TQ,
Wilson AM, Moore AL, Strand SE, Gordon MP, Doty SL (2002)
Expression of functional mammalian P450 2E1 in hairy root
cultures. Biotechnol Bioeng 77: 462-466
Bhadra R, Wayment DG,
Williams RK, Barman SN, Stone MB, Hughes JB, Shanks JV (2001)
Studies on plant-mediated fate of the explosives RDX and HMX.
Chemosphere 44: 1259-1264
Boominathan R, Doran PM
(2002) Ni-induced oxidative stress in roots of the Ni
hyperaccumulator, Alyssum bertolonii. New Phytol 156:
205-215
ADDIN EN.REFLIST
Boominathan R, Doran PM (2003a) Cadmium tolerance and
antioxidative defenses in hairy roots of the cadmium
hyperaccumulator, Thlaspi caerulescens. Biotechnol Bioeng
83: 158-167
Boominathan R, Doran PM (2003b) Organic acid complexation, heavy
metal distribution and the effect of ATPase inhibition in hairy
roots of hyperaccumulator plant species. J Biotechnol 101:
131-146
Boominathan R, Saha-Chaudhury NM, Sahajwalla V, Doran PM (2004)
Production of nickel bio-ore from hyperaccumulator plant
biomass: applications in phytomining. Biotechnol Bioeng 86:
243-250
Coniglio MS, Busto VD, González PS, Medina MI, Milrad S,
Agostini E (2008) Application of Brassica napus hairy
root cultures for phenol removal from aqueous solutions.
Chemosphere 72: 1035-1042
Cuello JL and Yue LC (2008) Ebb-and-Flow Bioreactor Regime and
Electrical Elicitation: Novel Strategies for Hairy Root
Biochemical Production. Electronic J Integrative Biosciences,
this issue
de Araujo BS, Charlwood VB, Pletsch M (2002) Tolerance and
metabolism of phenol and chloroderivatives by hairy root
cultures of Daucus carota L. Environ Pollut 117: 329-335
de Araujo BS, de Oliveira JO, Machado SS, Pletsch M (2004)
Comparative studies of peroxidases from hairy roots of Daucus
carota, Ipomea batatas and Solanum aviculare. Plant
Sci 167: 1151-1157
de Araujo BS, Dec J, Bollag JM, Pletsch M (2006) Uptake and
transformation of phenol and chlorophenols by hairy root
cultures of Daucus carota, Ipomoea batatas and
Solanum aviculare. Chemosphere 63: 642-651
Doty SL, James CA, Moore AL, Vajzovic A, Singleton GL, Ma C,
Khan Z, Xin G, Kang JW, Park JY, Meilan R, Strauss SH, Wilkerson
J, Farin F, Strand SE (2007) Enhanced phytoremediation of
volatile environmental pollutants with transgenic trees. Proc
Natl Acad Sci USA 104: 16816-16821
Doty SL (2008) Enhancing phytoremediation through the use of
transgenics and endophytes. New Phytol 179: 318-333.
Eapen S, Suseelan KN, Tivarekar S, Kotwal SA, Mitra R (2003)
Potential for rhizofiltration of uranium using hairy root
cultures of Brassica juncea and Chenopodium
amaranticolor. Environ Res 91: 127-133
Gavrilkescu M, Pavel LV, and Cretescu I (2008) Characterization
and remediation of soils contaminated with uranium. J Hazard
Mater, doi:10.1016/j.hazmats.2008.07.103
Georgiev MI, Pavlov AI, Bley T (2007) Hairy root type plant in
vitro systems as sources of bioactive substances. Appl Microbiol
Biotechnol 74: 1175-1185
González PS, Capozucca CE, Tigier HA, Milrad SR, Agostini E
(2006) Phytoremediation of phenol from wastewater, by
peroxidases of tomato hairy root cultures. Enzyme Microbial
Technol 39: 647-653
Guillon S, Trémeouillax-Guiller J, Pati PK, Rideau M, Gantet P
(2006) Hairy root research: Recent scenario and exciting
prospects. Curr Opin Plant Biol 9: 341-346
Gujarathi NP, Haney BJ, Park HJ, Wickramasinghe SR, Linden JC
(2005) Hairy roots of Helianthus annuus: a model system
to study phytoremediation of tetracycline and oxytetracycline.
Biotechnol Prog 21: 775-780
Gujarathi NP, Linden JC (2005) Oxytetracycline inactivation by
putative reactive oxygen species released to nutrient medium of
Helianthus annuus hairy root cultures. Biotechnol Bioeng
92: 393-402
Hajeb P, Selemat J, Ismail A, Abu Bakar F, Bakar J, Lioe HN
(2008) Hair mercury level of coastal communities in Malaysia: A
linkage with fish consumption. Eur Food Res Technol 227:
1349-1355
Harris RC, Rudd JWM, Amyot M, Babiarz CL, Beaty KG, Blanchfield
PJ, Bodaly RA, Branfireun BA, Gilmour CC, Graydon JA, Heyes A,
Hintelmann H, Hurley JP, Kelly CA, Krabbenhoft DP, Lindberg SE,
Mason RP, Paterson MJ, Podemski CL, Robinson A, Sandilands KA,
Southworth GR, St. Louis VL, Tate MT (2007) Whole-ecosystem
study shows rapid fish-mercury response to changes in mercury
deposition. Proc Natl Acad Sci USA 104: 16586-16591
Hatcher JM, Delea KC, Richardson JR, Pennell KD, Miller GW
(2008) Disruption of dopamine transport by DDT and its
metabolites. Neurotoxicol 29: 682-690
Kučerova P, Macková M, Chromá L, Burkhard J, Tříska J, Demnerová
K, Macek T (2000) Metabolism of polychlorinated biphenyls by
Solanum nigrum hairy root clone SNC-90 and analysis of
transformation products. Plant Soil 225: 109-115
Kuiper I, Lagendijk EL, Bloemberg GV, Lugtenberg BJ (2004)
Rhizoremediation: a beneficial plant-microbe interaction. Mol
Plant Microbe Interact 17: 6-15
Macková M, Macek T, Kučerová P, Burkhard J, Pazlarová J,
Demnerová K (1997a) Degradation of polychlorinated biphenyls by
hairy root culture of Solanum nigrum. Biotechnol Lett 19:
787-790
Macková M, Macek T, Ocenaskova J, Burkhard J, Demnerová K,
Pazlarová J (1997b) Biodegradation of polychlorinated biphenyls
by plant cells. Int Biodeterior Biodegrad 39: 317-325
McAfee BJ, White EE, Pelcher LE, Lapp MS (1993) Root Induction
in pine (Pinus) and larch (Larix) sp. using
Agrobacterium rhizogenes. Plant Cell Tissue Organ Cult 34:
53-62.
Nedelkoska TV, Doran PM (2000a) Characteristics of heavy metal
uptake by plant species with potential for phytoremediation and
phytomining. Minerals Eng 13: 549-561
Nedelkoska TV, Doran PM (2000b) Hyperaccumulation of cadmium by
hairy roots of Thlaspi caerulescens. Biotechnol Bioeng
67: 607-615
Nedelkoska TV, Doran PM (2001) Hyperaccumulation of nickel by
hairy roots of Alyssum species: Comparison with whole
regenerated plants. Biotechnol Prog 17: 752-759
Pilon-Smits E (2005) Phyotoremediation. Annu Rev Plant Biol 56:
15-39
Rentz JA, Alvarez PJJ, Schnoor JL (2005) Benzo [a]pyrene
co-metabolism in the presence of plant root extracts and
exudates: Implications for phytoremediation. Environ Pollut 136:
477-484
Rezek J, Macek T, Macková M, Tříska J (2007) Plant metabolites
of polychlorinated biphenyls in hairy root culture of black
nightshade Solanum nigrum SNC-90. Chemosphere 69:
1221-1227
Sadasivaiah S, Tozan Y, Breman JG (2007)
Dichlorodiphenyltrichloroethylyne (DDT) for indoor residual
spraying in Africa: How can it be used for malaria control?. Am
J Trop Med Hyg 77: 249-263
Singh S, Melo JS, Eapen S, D' Souza SF (2006) Phenol removal
using Brassica juncea hairy roots: Role of inherent
peroxidase and H2O2. J Biotechnol 123:
43-49
Srivastava S, Srivastava AK (2007) Hairy root culture for
mass-production of high-value secondary metabolites. Crit Rev
Biotechnol 27: 29-43
Suresh B, Ravishankar GA (2004) Phytoremediation- a novel and
promising approach for environmental clean-up. Crit Rev
Biotechnol 24: 97-124
Suresh B, Sherkhane PD, Kale S, Eapen S, Ravishankar GA (2005)
Uptake and degradation of DDT by hairy root cultures of
Cichorium intybus and Brassica juncea. Chemosphere
61: 1288-1292
Tamaoki M, Freeman JL, Pilon-Smits EAH (2008) Cooperative
ethylene and jasmonic acid signaling regulates selenite
resistance in Arabidopsis. Plant Physiol 146: 1219-1230
Van Aken B (2008) Transgenic plants for phytoremediation:
helping nature to clean up environmental pollution. Trends
Biotechnol 26: 225-227
Veena V, Taylor CG (2007) Agrobacterium rhizogenes:
Recent developments and promising applications. In Vitro Cell
Dev Biol 43: 383-403
Villarreal ML, Caspeta L, and Quintero-Ramirez R (2008) From the
Mayan highlands to the bioreactors: In vitro tissue
culture of the Mexican Medicinal plant Solanum chrysotrichum.
Electronic J Integrative Biosciences, this issue
Vinterhalter B, Savić J, Platiša J, Raspor M, Ninković S, Mitić
N, Vinterhalter D (2008) Nickel tolerance and hyperaccumulation
in shoot cultures regenerated from hairy root cultures of
Alyssum murale Waldst et Kit. Plant Cell Tis Organ Cult 94:
299-303
Walker TS, Bais HP,
Halligan KM (2003) Metabolic profiling of root exudates of
Arabidopsis thaliana. J Agric Food Chem 51: 2548-2554
Weathers P, Liu C, Towler M, and Wyslouzil B (2008) Mist
reactors: Principle, comparison of various systems, and case
studies. Electronic J Integrative Biosciences, this issue
Wevar-Oller AL, Agostini E, Talano MA, Capozucca C, Milrad SR,
Tigier HA, Medina MI (2005) Overexpression of a basic peroxidase
in transgenic tomato (Lycopersicon esculentum Mill. cv.
Pera) hairy roots increases phytoremediation of phenol. Plant
Sci 169: 1102-1111
Xingmao M, Burken JG (2003) TCE Diffusion to the atmosphere in
phytoremediation applications. Environ Sci Technol 37: 2534-2539