|
Research Article
Wavelength
Discrimination in the Zebrafish (Danio rerio):
Evidence for Functional Color Vision
Tim Thornberry, Jr., Michael Risner and Steven J. Haggbloom
Western Kentucky University
Address correspondence to Steven J. Haggbloom, Ph.D.,
Department of Psychology, Western Kentucky University, 1906
College Heights Blvd., #21030, Bowling Green, KY 42101-1030,
e-mail:
steven.haggbloom@wku.edu
ABSTRACT:
The zebrafish (Danio rerio) is a popular vertebrate model in
several fields of research, especially visual neuroscience,
where it has been used for anatomical, physiological, genetic,
developmental, and behavioral research. Anatomical and
physiological studies have shown the zebrafish has the necessary
mechanisms for color vision, but it is not known whether
zebrafish can use color vision to regulate behavior. Recently,
studies have shown that zebrafish can learn an instrumental
discrimination task. The study reported here used instrumental
discrimination learning procedures with wavelength as the
discriminanda. The results indicate that the zebrafish does,
indeed, have functional color vision. The methods used here
could be further developed to investigate the functionality of
UV visual processing in zebrafish, color perception thresholds,
and similar phenomena.
The zebrafish, because of numerous
advantageous characteristics, has come to be used extensively as the
vertebrate model of choice in many areas of research. Its
advantageous characteristics include transparent chorions, which
allow for unobtrusive observation of the developing embryo, the
capacity to maintain a large subject pool due to prolific breeding
and rapid development, and general hardiness, making the zebrafish
an economical, easy-to-maintain subject. The zebrafish is an ideal
vertebrate model for visual neuroscience because it has a retinal
anatomy and physiology similar to that of other vertebrates so that
the results of research on zebrafish can be generalized to other
vertebrates, including humans.
Some of the most useful data from
zebrafish have been obtained when anatomical, physiological, or
genetic procedures were combined with behavioral methods. For
example, Taylor, Hurley, Van Epps, and Brockerhoff (2004) used
behavioral genetic screens to show that a deficit in pyruvate
dehydrogenase (PHD, a normally lethal condition due to abnormal
mitochondrial metabolism), could be countered by adding ketogenic
substrates to the housing water, a result with implications for the
treatment of PHD and other congenital diseases that affect early
embryonic development in humans. Darland and Dowling (2001) combined
behavioral techniques with genetic mutations to identify zebrafish
with decreased sensitivity to cocaine. They suggested that such
studies could potentially identify specific genes associated with
addiction. Muto et al. (2005) combined genetic mutations with
psychophysical measurements to show the effectiveness of using
mutant zebrafish in identifying specific genes associated with
visual functioning. Ren, McCarthy, Zhang, Adolph, and Li (2002) also
combined genetic mutations with behavioral measures and found that
retinal screening pigments help regulate behavioral responses in
zebrafish. Finally, Page-McCaw et al. (2004) combined genetic and
physiological data with optokinetic behavioral data to study light
adaptation in zebrafish.
Recently, Bilotta, Risner, Davis, and
Haggbloom (2005) suggested that more behavioral techniques need to
be developed to fully realize the potential of the zebrafish as a
vertebrate model for visual neuroscience. To that end, they
developed procedures for investigating instrumental choice
discrimination learning in zebrafish. In their task, subjects were
rewarded for swimming into a chamber lit by a white-light stimulus
(the positive discriminative cue, S+) and received no reward for
entering a dark chamber (the negative discriminative cue, S-), a
stimulus arrangement in opposition to the natural tendency of
zebrafish to prefer a dark environment. They reported that the
zebrafish learned this discrimination to a criterion of at least 80%
correct. Colwill, Raymond, Ferreira, and Escudero (2005) also
reported evidence of instrumental discrimination learning in
zebrafish.
In two of the experiments reported by
Colwill et al. (2005), the S+ and S- discriminanda were colored
sleeves (purple vs. green or blue vs. red) fitted over the arms of a
T-maze. However, there was no control for possible differences in
brightness between the discriminanda. Consequently, it is possible
that color differences were confounded with brightness differences.
If the natural zebrafish preference for a darker environment also
manifests in a preference for darker stimuli, the functional
discriminanda in the Colwill et al. experiments could have been
brightness rather than color. To date, there have been no other
investigations of color vision-regulated discrimination learning in
zebrafish.
The purpose of this experiment was to
investigate the capacity of the zebrafish to learn an instrumental
discrimination task with differently colored but equally luminant
lights as the S+ and S- discriminanda. To equate the discriminanda
on luminance, idiosyncratic isoluminant values were behaviorally
determined for each fish for two monochromatic stimulus lights.
Those light were then used as the S+ and S- cues in an instrumental
discrimination learning task modeled after that used by Bilotta et
al. (2005).
METHOD
Subjects
Eight adult (> 1 yr.) male and female
zebrafish were used in this experiment. The fish were purchased from
a local pet store and housed in an aquarium housing system (Aquaneering
Incorporated, San Diego, CA) which maintained a water temperature of
28°
to 30°C,
a pH of 6.8 to 7.2, and a light cycle of 14 hours on and 10 hours
off. Fish were housed individually for at least 2 weeks prior to
the start of conditioning procedures in order to accustom each
zebrafish, a naturally schooling fish, to being alone and to provide
a means of identifying each fish. This was done because fish in the
present study were trained individually rather than in groups. All
fish were approximately the same size. These procedures were adapted
from those used by Bilotta et al. (2005).
Behavioral Apparatus
The behavioral apparatus, shown in
Figure 1A, was the same modified 19 L fish aquarium used by Bilotta
et al. (2005). The apparatus was divided into three areas: a
reservoir area, a home area, and a chamber area. The reservoir area
was divided from the home area by a removable divider, which
restricted an individual subject’s movement to the home area and
chamber area. A removable heater was placed in the reservoir area to
help maintain a water temperature of 25°
to 29°C
during all conditioning procedures. The subjects remained in the
home area between trials. A gate stabilizer divided the home and
chamber areas and held an adjustable gate (see Figure 1B) which
could be raised and lowered to permit or prevent a fish from
accessing the chamber or home areas. The gate had three “portholes”
through which the fish could view the visual stimuli presented in
the chamber area while still being confined to the home area.
Although the chamber area was divided into three separate units, the
middle chamber was always blocked and only the two side units were
used in this experiment. A liquid light-guide holder was placed
outside the chamber area of the apparatus (Figure 1A).
Prior to the start of a session of
data collection, the apparatus was filled with 4 L of conditioned
water taken from the fish-housing system.
Optical System
Monochromatic visual stimuli were
produced by two light sources. A 500nm stimulus was always produced
by a 150-W xenon arc lamp (Model LH 150, Spectral Energy, Westwood,
NJ). The light was collimated, passed through a water bath, and
focused by a lens onto a shutter (Model LS62M2, Uniblitz, Rochester,
NY) that was controlled by a shutter driver (Model D122, Uniblitz,
Rochester, NY). An interference filter (half bandwidth of 10 nm,
Oriel, Stratford, CT) was used to filter the white light of the arc
lamp to produce a 500 nm stimulus wavelength. Stimulus luminance was
controlled by neutral density filters (Model 398, Reynard, San
Clemente, CA). The 500 nm stimulus was then focused onto a liquid
light guide (Model 77556, Oriel), which was directed into the
selected chamber.
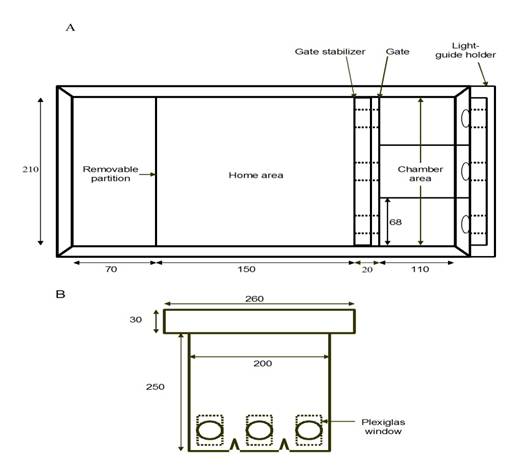
Fig 1. Schematic of the behavioral apparatus. Details can be found in
Bilotta et al. (2005). (A) Top view. (B) Side view of
the removable gate.
The second light stimulus was
produced by a halogen light (World Precision Instruments, Sarasota,
FL) passed through a liquid light guide (World Precision
Instruments, Model SI-72-8, Sarasota, FL). The light was passed
through interference filters (half bandwidth of 10 nm, Oriel,
Stratford, CT) that produced either a 460 or 540 nm monochromatic
stimulus. This light was then aimed at another liquid light guide
(World Precision Instruments, Model SI-72-8, Sarasota, FL) that was
directed into the second chamber. Stimulus luminance from this light
source was adjusted via a rotary dimmer attached to the light
source. A 50-W tungsten lamp (Model 1575, Underwriters Laboratories,
Northbrook, IL) was placed above the behavioral apparatus in order
to produce a 2 lux background illuminance.
Procedures
There were five distinct training
phases in this experiment. These were: habituation, chamber-entry
training, stimulus-association training, isoluminance training, and
wavelength-discrimination training.
During training, the subjects’ diets
were restricted to a small amount of flake food daily. The training
procedures were adapted from those used by Bilotta et al. (2005).
Habituation
After subjects’ diets were restricted
to a small amount of flake food each day for two days,
apparatus-habituation training commenced. Habituation training,
consisting of one session per day over two consecutive days, was
used to familiarize the subjects with the behavioral apparatus.
During each session, the room lights were turned off, and a
background light of 2 lux was present. Each fish was individually
placed into the home area of the behavioral apparatus, and the gate
was raised to allow the subject access to the chamber areas. The
fish was allowed to swim freely in the apparatus for 20 min. After
this time, the session was terminated, the gate was lowered to
restrict the subject’s movement to the home chamber, the room lights
were turned on, and the subject was removed from the behavioral
apparatus and placed back into its individual container in the
housing system.
Chamber-Entry Training
Immediately following habituation
training, each fish received one 20-trial session of chamber-entry
training daily for three consecutive days. At the beginning of each
chamber-entry training session, the subject was re-habituated to the
apparatus for 5 min. Following habituation, and while the fish was
in the home area, the gate was lowered. After 10 s, the gate was
raised, allowing the subject to swim into one of the two chambers.
If the subject swam into one of the chambers, the gate was lowered
to restrict the subject to the chamber it chose. One of the three
monochromatic stimuli (460, 500, or 540 nm) was then presented in
conjunction with a food reward of 5-10 live brine shrimp
administered with a glass eye dropper. The fish was given 30 s to
consume the brine shrimp. The visual stimulus was then terminated,
the gate was raised, and the fish was allowed to swim back into the
home area. The gate was then lowered, marking the end of the trial.
After a 10-s intertrial interval (ITI), a new trial began. In the
event that a subject did not swim into one of the two chambers after
90 s, the gate was lowered and the trial was terminated. At the end
of the session, the subject was returned to the housing system. Fish
that did not enter one of the two chambers on all 20 trials in the
last training session were replaced.
Stimulus-Association Training
After chamber-entry training
concluded, subjects began stimulus-association training. Again,
subjects were habituated to the apparatus for 5 min, and then
confined to the home area. The monochromatic stimulus later to be
used as S+ was then presented in one of the two chamber areas for 10
s (this was the 460nm light for two fish, the 500nm light for four
fish, and the 540nm light for the remaining two fish). The gate was
then raised, and the subject was allowed to swim into either the
illuminated or the dark chamber. If the subject swam into the
illuminated S+ chamber area, this was scored as a correct response.
The gate was then lowered, restricting the subject’s movement to
that chamber, the subject was reinforced with a live, brine shrimp
food reward, and it was allowed 30 s to consume the food.
Afterwards, the visual stimulus was terminated, the gate was raised,
and the fish was allowed back into the home area ending the trial.
If the subject swam into the dark chamber area, the gate was
lowered, the visual stimulus was terminated, and the subject was
confined to the dark chamber area for 30 s without food
reinforcement. The gate was then raised and the subject was allowed
back into the home area ending the trial. If the subject failed to
choose either of the two chambers after 90 s, the visual stimulus
was terminated, the gate was lowered, and the subject remained in
the home area until a new trial began. Each stimulus-association
training session consisted of 20 trials separated by a 10-s ITI. A
quasi-random process was used to designate a chamber as S+, and each
chamber was designated S+ for 10 of the 20 trials to prevent
development of a chamber preference. At the end of the 20 trials,
the subject was removed from the apparatus and returned to the
housing system. Each fish was trained to a criterion of 80% correct
responses per session for two consecutive sessions.
Isoluminance Training
The purpose of this experiment was to
determine whether zebrafish could learn an instrumental
discrimination with different wavelengths of light as the
discriminative cues. Isoluminance training was used to determine
luminance values at which the S+ stimulus, associated with a food
reward in the previous training phase, and a second monochromatic
stimulus that would serve as the S- cue during discrimination
training, were perceived as equally bright. By determining these
isoluminant values, we eliminated any potential confound between
color and brightness. By identifying idiosyncratic isoluminant
values, as opposed to a single isoluminant point for each pair of
wavelengths, we also controlled for the possibility that the
perception of brightness could differ among subjects.
The methodology used for isoluminance
training was essentially the same as that used for
stimulus-association training. However, in these sessions, the
previously dark chamber now contained the monochromatic stimulus to
be used as S- during discrimination learning. Table 1 shows the
stimulus combinations used as S+ and S- for each fish.
After 5 min of habituation, the
subject was confined to the home area by lowering the gate. The S+
and S- stimuli were then presented simultaneously. After 10 sec, the
gate was raised and the subject was allowed to swim into either the
S+ or S- chamber. In the event the subject entered the S+ chamber,
the gate was lowered, the S- cue was terminated, and the subject was
rewarded with 5-10 live brine shrimp. After 30 s of feeding, the
gate was raised, the subject was allowed back into the home area,
and the gate was lowered. If the subject entered the S- chamber,
both stimuli were terminated and the fish was confined to the S-
chamber for 30 s without food reinforcement. The gate was then
raised, allowing the subject to return to the home area. If the fish
did not enter a chamber after 90 s of swimming in the home area, the
trial was terminated by turning the stimuli off, lowering the gate,
and confining the fish in the home area until the next trial. All
trials were separated by a 10-s ITI.
The isoluminance point for each pair
of stimuli for each fish was determined by varying the illuminance
of the 500 nm stimulus between trials in steps of 0.3 log units of
attenuation. Six different illuminance values were tested per
session.
Each isoluminance training session
included 30 trials. Both the S+ chamber and the illuminance of the
500nm stimulus varied in a quasi-random fashion, with each chamber
designated as S+ for 15 of the 30 trials. Each of the 6 illuminance
values for the 500 nm stimulus was presented 5 times per session.
Isoluminance training continued until an isoluminant point was
determined, defined as the attenuation at which the average
percent-correct response fell closest to chance levels (50%).
Wavelength-Discrimination Training
After isoluminance training
determined the subject’s isoluminant point for the two monochromatic
stimuli, the subject began wavelength discriminatio training. During
these sessions, the illuminance of the 500 nm stimulus was fixed at
the isoluminant value determined during isoluminance training,
otherwise the training methodology was essentially the same as that
used for isoluminance training, and the stimuli designated as S+ and
S- were the same as in isoluminance training.
A trial began with the subject in the
home area. The gate was then raised and the subject was allowed to
swim into one of the two chamber areas. If the fish entered the S+
chamber, the gate was lowered, the S- was terminated, and the
subject was rewarded with a food reward of 5-10 live brine shrimp.
If the fish chose the S- chamber, the gate was closed, the stimuli
were terminated, and the subject remained in the dark chamber for 30
s without food reinforcement. After 30 s confinement to either the
S+ or S- chamber, the gate was raised and the subject was allowed to
reenter the home area. The gate was then lowered, and an ITI of 10 s
passed before a new trial began. If the subject refused to swim into
either chamber within 90 s of trial initiation, the stimuli were
terminated, the gate was lowered, and a new trial began after a 10-s
ITI. Subjects received two consecutive 10-trial sessions per day
until they reached a criterion of 80% correct on two consecutive
sessions.
RESULTS
Stimulus-Association Training
All eight fish learned to enter the
chamber illuminated by the S+ stimulus. Figure 2 shows the mean
percent correct for all fish across 14 training sessions. Because
training was terminated for each fish after the criterion was
reached, the graph reflects an assigned score of 80% correct for
that fish for the remaining sessions. Error bars represent
±
1 standard deviation. Variability was relatively high until the 7th
training session, after which there was very little variability
because only one subject (Z9) had not yet reached the 80% correct
criterion (dashed line). On average, it took subjects 6.75 sessions
to reach the learning criterion. If the data for fish Z9 are
excluded, the learning criterion was reached in an average of 5.71
sessions. All subjects satisfied the learning criterion within 14
sessions. Figure 3 shows individual learning curves for each fish.
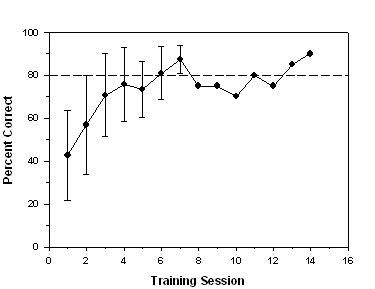
Fig 2. Mean percent correct for all eight fish over 14 sessions of
stimulus-association training.
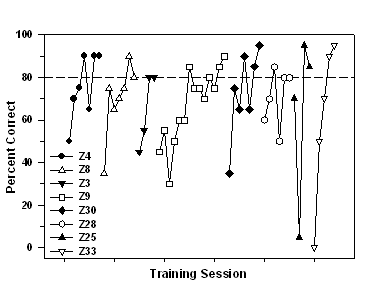
Fig 3. Individual learning curves for each fish during stimulus-association
training
Isoluminance Training
Figures 4-11 show the results of
isoluminance training separately for each subject. In all figures,
the X-axis is log-stimulus attenuation and the Y-axis is
percent-correct response for each irradiance value with the dashed
line representing chance performance. Error bars represent
±
1 standard error of the mean. The isoluminant point was defined as
the attenuation of the 500nm stimulus at which the average
percent-correct fell closest to chance (arrow).
Comparing across figures, it can be
seen that isoluminant values varied among subjects given the same
wavelength stimuli as discriminanda. For example, subjects Z4 and Z8
experienced the 500 nm stimulus as S+ and the 460nm stimulus as S-.
The performance of subject Z4 was nearest chance (47.86%, Figure 4)
when -1.5 log units of attenuation were applied to the 500 nm S+
stimulus, whereas, for subject Z8 the isoluminant point occurred at
-0.6 log units of attenuation (57.5%, Figure 5). Subjects Z3 and Z9
experienced the 500 nm stimulus as S+ and the 540 nm stimulus as S-.
As can be seen in Figures 6 and 7, the isoluminant point for subject
Z3 occurred at -1.5 log units of attenuation while the isoluminant
point for subject Z9 occurred at -1.2 log units of attenuation.
Subjects Z30 and Z28 experienced the 460 nm stimulus as S+ and the
500 nm stimulus as S-. As can be seen in Figures 8 and 9, the
isoluminant point for subject Z30 occurred at -0.6 log units of
attenuation while the isoluminant point for subject Z28 occurred at
an attenuation of -0.9 log units. Finally, subjects Z25 and Z33
experienced the 540 nm stimulus as S+ and the 500 nm stimulus as S-.
Figures 10 and 11 show that the isoluminant point for subject Z25
occurred at -0.3 log units of attenuation while the isoluminant
point for subject Z33 occurred at -0.6 log units of attenuation.
This pattern of results confirms the potential importance of using
idiosyncratic isoluminance values for wavelength-discrimination
training.
Wavelength-Discrimination Training
Figure 12 shows the
wavelength-discrimination learning acquisition curves for each fish.
The X-axis represents training session and the Y-axis represents
percent correct responses. The dashed line represents the learning
criterion of 80% correct, and the dotted line represents chance. As
can be seen, all subjects reached criterion, although after
different amounts of training. Subjects took an average of 6.88
sessions to reach the learning criterion, and all subjects reached
criterion within 16 sessions.
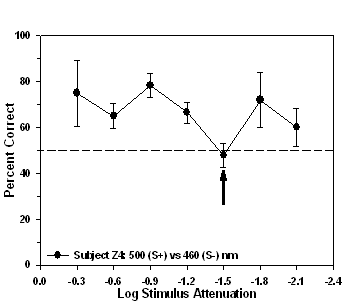
Fig 4. Isoluminance training results and isoluminant point for subject Z4
trained to approach a 500 nm (S+) stimulus during
stimulus-association training.
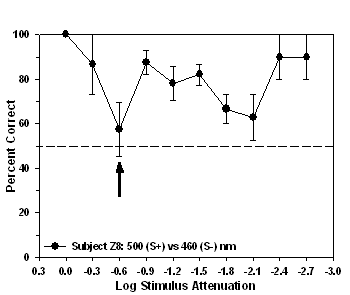
Fig 5. Isoluminance training results and isoluminant point for subject Z8
trained to approach a 500 nm (S+) stimulus during
stimulus-association training
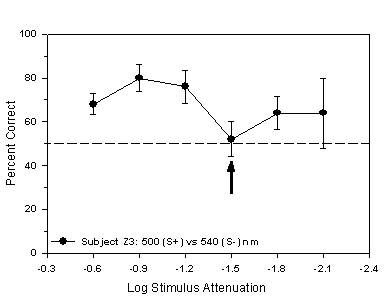
Fig 6.
Isoluminance training results and isoluminant point for subject Z3
trained to approach a 500 nm (S+) stimulus during
stimulus-association training.
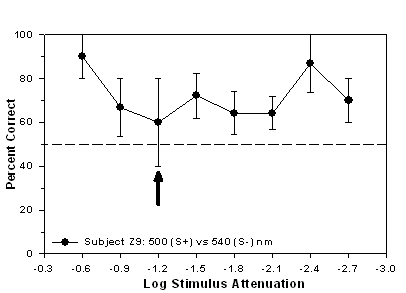
Fig 7.
Isoluminance training results and isoluminant point for subject Z9
trained to approach a 500 nm (S+) stimulus during
stimulus-association training.
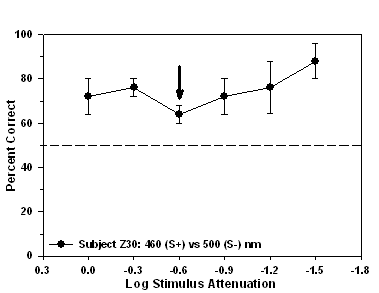
Fig 8. Isoluminance training results and isoluminant point for subject Z30
trained to approach a 460 nm (S+) stimulus during
stimulus-association training.
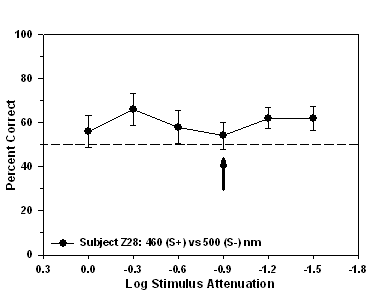
Fig 9. Isoluminance training results and isoluminant point for subject Z28
trained to approach a 460 nm (S+) stimulus during
stimulus-association training.
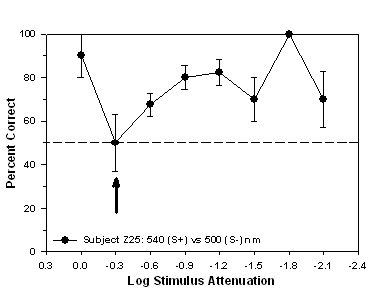
Fig 10.
Isoluminance training results and isoluminant point for subject
Z25 trained to approach a 540 nm (S+) stimulus during
stimulus-association training.
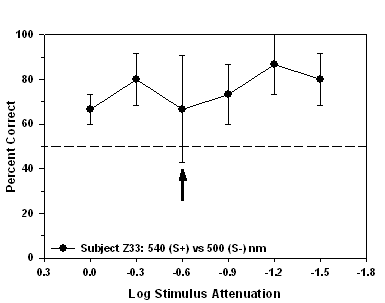
Fig 11.
Isoluminance training results and isoluminant point for subject Z33
trained to approach a 540 nm (S+) stimulus during
stimulus-association training.
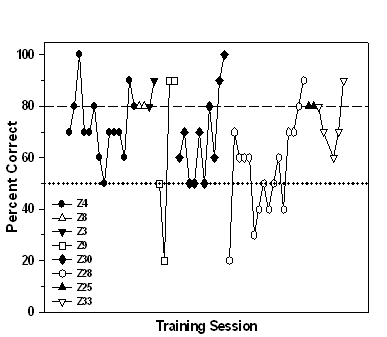
Fig 12.
Individual learning curves for wavelength-discrimination training.
DISCUSSION
Stimulus-Association Training
The present study supports the
findings of Bilotta et al. (2005) and Colwill et al. (2005) in
demonstrating that the zebrafish can learn a relatively difficult
appetitive instrumental discrimination learning problem. All
subjects in the present study were able to associate a monochromatic
visual stimulus with a food reward by overcoming their inherent
preference for dark environments over lit environments. As was seen
in Bilotta et al.’s (2005) study, there was considerable variability
across fish in the number of sessions required to reach the learning
criterion.
The main purpose of this experiment
was to determine if the zebrafish is capable of using color
(wavelength) information to regulate behavior (i.e., to determine
whether the zebrafish has functional color vision). Although Colwill
et al. (2005) used color cues as the putative discriminanda, they
did not control for the possibility that color differences between
discriminanda were confounded with brightness differences. In order
to eliminate possible brightness differences between the
discriminanda and help ensure that color was the functional
discriminative stimulus dimension in the present experiment, we
identified idiosyncratic isoluminant values for the S+ and S-
wavelength stimuli. The isoluminant point was defined as the degree
of attenuation of the 500 nm stimulus that resulted in nearly chance
discrimination between it and another monochromatic stimulus. At the
isoluminant point, which was determined prior to wavelength
discrimination training; it was assumed that the subject could no
longer use brightness cues to differentiate between the S+ and S-
cues. While it is impossible to know if the isoluminant stimuli were
actually perceived by a subject to be equally bright, the
isoluminance training ensured the stimuli were functionally
equivalent. Furthermore, the use of all three monochromatic stimuli
in different combinations as both S+ and S- (across different fish)
controlled for a possible innate tendency to approach a certain
color, and also countered any possible brightness preference that
might remain after the isoluminant points were determined.
The identification of individual
isoluminant values for all subjects showed that the isoluminant
values varied between subjects tested with the same pair of
discriminanda. This finding confirms the potential importance of
using idiosyncratic isoluminant values to control for potential
brightness differences between discriminanda. The results obtained
here with idiosyncratic isoluminant discriminanda of different
wavelengths show that zebrafish can, indeed, learn an appetitive
instrumental discrimination problem with color as the functional
discriminanda. These results are consistent with a conclusion that
the zebrafish has functional color vision as would be expected given
its retinal anatomy.
Future studies of zebrafish vision
and visual perception can be performed using the procedure used
here. Such research should determine whether wavelength
discrimination is possible at wavelengths other than those used
here. The present study only investigated discrimination abilities
at 460, 500, and 540 nm wavelengths. These wavelengths were chosen
based on Risner, Bilotta, Vukmanic, and Moore’s (2006) study, which
determined behavioral spectral sensitivity thresholds for zebrafish.
In the Risner et al. study, zebrafish were most sensitive to
monochromatic stimuli of 500 nm wavelength. Also, they found that
zebrafish were relatively insensitive to wavelengths of 460 and 540
nm. The present study sought to determine if wavelength
discrimination was possible at all in zebrafish. Had the present
study used wavelengths that were relatively the same in spectral
sensitivity, it may have been more difficult to determine if color
discrimination was possible in zebrafish. Further studies could also
use this paradigm to determine visual stimulus-generalization
thresholds in zebrafish by using wavelengths of monochromatic light
that differ by less than 40 nm, the wavelength differences used in
this study. The zebrafish’s unique ability to see UV light could
also be studied, as future studies using this paradigm could examine
wavelength-discrimination abilities of zebrafish in the UV spectrum,
an examination that has yet to be performed. Combining such
threshold information with pharmacological and genetic techniques
may help determine the effects certain drugs and mutations have on
visual perceptual abilities as measured by psychophysical
techniques. Such studies could lead to the development of new models
for vertebrate visual deficits such as color blindness and night
blindness.
Acknowledgements
This study was
funded by the National Center for Research Resources (NCRR) Grant
NIH:NCRR P20RR16481 to Joseph Bilotta.
REFERENCES
Bilotta, J., Risner, M. L., Davis, E.
C., & Haggbloom, S. J. (2005). Assessing appetitive choice
discrimination learning in zebrafish. Zebrafish, 2,
259-268.
Bilotta, J., Thornberry, Jr., T., &
Saszik, S. (2008). Dark-adaptation functions of the developing
zebrafish. Unpublished manuscript.
Branchek, T., & Bremiller, R. (1984).
The development of photoreceptors in the zebrafish, Brachydanio
rerio. I. Structure. The Journal of Comparative Neurology,
224, 107-115.
Colwill, R. M., Raymond, M. P.,
Ferreira, L., & Escudero, H. (2005). Visual discrimination learning
in zebrafish (Danio rerio). Behavioural Processes, 70,
19-31.
Darland, T. & Dowling, J. E. (2001).
Behavioral screening for cocaine sensitivity in mutagenized
zebrafish. Proceedings of the National Academy of Sciences of the
United States of America, 98, 11691-11696.
Guo, S. (2004). Linking genes to
brain, behavior and neurological diseases: What can we learn from
zebrafish? Genes, Brain and Behavior, 3, 63-74.
Muto, A., Orger, M. B., Wehman, A. M.,
Smear, M. C., Kay, J. N., Page-McCaw, P. S., Gahtan, E., Xiao, T.,
Nevin, L. M., Gosse, N. J., Staub, W., Finger-Baier, K., & Baier, H.
(2005). Forward genetic analysis of visual behavior in zebrafish.
Public Library of Science Genetics, 1, 575-588.
Page-McCaw, P. S., Chung, S. C., Muto,
A., Roeser, T., Staub, W., Finger-Baier, K. C., Korenbrot, J. I., &
Baier, H. (2004). Retinal network adaptation to bright light
requires tyrosinase. Nature Neuroscience, 7,
1329-1336.
Ren, J. Q., McCarthy, W. R., Zhang,
H., Adolph, A. R., & Li, L. (2002). Behavioral visual responses of
wild-type and hypo pigmented zebrafish. Vision Research,
42, 293-299.
Risner, M. L., Bilotta, J., E.,
Vukmanic, E. V., & Moore, A. (2006). Behavioral spectral sensitivity
of the zebrafish (Danio rerio). Vision Research, 46,
2625-2635.
Russell, C. (2003). The roles of
hedgehogs and fibroblast growth factors in eye development and
retinal cell rescue. Vision Research, 43, 899-912.
Taylor, M. R., Hurley, J. B., Van
Epps, H. A., & Brockerhoff, S. E. (2004). A zebrafish model for
pyruvate dehydrogenase deficiency: Rescue of neurological
dysfunction and embryonic lethality using a ketogenic diet.
Proceedings of the National Academy of Sciences of the United States
of America, 101, 4584-4589.
Tropepe, V. & Sive, H. L. (2003). Can
zebrafish be used as a model to study the neurodevelopment causes of
autism? Genes, Brain and Behavior, 2, 268-281.
Accepted for
publication: 10 December 2008
|