From the Mayan Highlands to the Bioreactors: In Vitro
Tissue Culture of the Mexican Medicinal Plant
Solanum chrysotrichum
María
Luisa Villarreal1*, Luis Caspeta1, and
Rodolfo Quintero-Ramírez2
1Centro
de Investigación en Biotecnología, Universidad Autónoma del
Estado de Morelos. Av. Universidad 1001, Col. Chamilpa.
Cuernavaca 62209 Morelos, México.
2División
de Ciencias Naturales e Ingeniería. Universidad
Autónoma Metropolitana-Cuajimalpa. Artificios 40, Col. Hidalgo.
México 01120 D.F.
*Corresponding author; email:
luisav@cib.uaem.mx
Keywords:
antimycotics, saponins, airlift reactors, natural products,
tinae
ABSTRACT
Solanum chrysotrichum
of the Solanaceae family was selected for investigation as
according to ethnomedical knowledge it represents the plant most
widely used by the Highland Maya from Chiapas, Mexico for the
treatment of skin mycosis. Research with a multidisciplinary
focus has been applied to study the pharmacological,
phytochemical, clinical and biotechnological aspects of this
plant species, and is reviewed here, in this paper. In vitro
pharmacological studies demonstrated the efficacy of this plant
for inhibiting the growth of dermatophytes (Trycophyton
mentagrophytes, T. rubrum and Microsporum gypseum)
in culture. Clinical tests were conducted and confirmed the
efficacy of plant extracts for treating patients suffering from
tinea pedis. Phytochemical studies achieved the isolation
and purification of the antimycotic principles, which were found
to be present in a family consisting of six novel spirostanol
saponins, designated as SC-1 to SC-6. In order to obtain higher
yields of the saponins, we applied a number of biotechnological
procedures including micropropagation and the establishment of
cell and hairy root cultures. Cell suspensions were scaled-up in
10 L airlift bioreactors. Novel fittings on 2 and 10 L airlift
reactors were designed and evaluated to up the scale of S.
chrysotrichum hairy roots, permitting the production of
higher yields of the most active saponins; SC-2 and SC-4. This
Mexican plant represents an important popular remedy, whose
cultivation in bioreactors was made possible for the first time.
Here we review the procedures involved in the bio-production of
antimycotic chemicals from the cells and hairy roots of the
plant on a larger scale.
IMPORTANCE OF THE
PLANT
A group of plants
commonly known as sosas are used among the Highland Maya of
Chiapas, Mexico, for the treatment of skin ailments and
dermatological infections. Traditional healers describe the
plant S. chrysotrichum (Schldl) from the Solanaceae
family, as the most effective herbal remedy for the treatment of
tinae (Tinae pedis), scabies and other mycosis (Zurita
and Zolla, 1986; Lozoya and Aguilar, 1987). Mayan ethnic groups
apply different names to this plant: kúx peul among the
Tzotzil, kúxbal chíx among the Tzeltal, and pajutiek
among the Chol.
Herbal medication
produced from this plant is normally prepared by boiling fresh
leaves in water and administering the solution topically as
plasters or poultices, but it is also sometimes prescribed as an
oral infusion (Zurita and Zolla, 1986). The plant is a perennial
herb which is able to grow up to 2 m in height and has spiny
stems. The leaves are rough to touch, 20-30 cm long and 10 cm
wide and covered with large hairs. The flowers are white with a
star-like appearance (Figure 1).

Figure 1.
Adult specimen of Solanum chrysotrichum (Schldl.).
IN VITRO
PHARMACOLOGICAL TESTING
Initial
pharmacological research concerning plants collected in Chiapas,
consisted of testing for their ability to inhibit the growth of
bacteria and dermatophytes in culture. Organic solvent extracts
were prepared from leaves and tested against Gram positive and
Gram negative bacteria, as well as against the yeast Candida
albicans and the dermatophyte Microsporum gypseu.
Promising activity against these last two microorganisms was
evident. When the extracts were specifically tested against the
main causative agents of athletes foot: T. mentagrophyts,
T. rubrum and M. gypseum, they exhibited
significant antifungal activity (Lozoya et al., 1991).
ISOLATION AND
PURIFICATION OF ANTIFUNGAL COMPOUNDS
A major bioactive
constituent, obtained from the methanolic extract prepared from
the leaves of S. chrysotrichum was purified by means of
bioassay guided fractionations using T. mentagrophytes as
the biological monitor. The molecular structure of this compound
designated as SC-1 was established on the basis of spectral
analyses, mainly proton and 13C-NMR, including
two-dimensional techniques, i.e. 1H-1H
COSY, HMQC and HMBC, and was identified as consisting of a novel
spirostanol saponin with glycoside moieties. The chemical
structure of SC-1 was established as 3-O-{β-D-quinovopyranosyl
(1→6)-β-D-glucopyranosyl (1→6)-β-D-glucopyranosyl} chlorogenin
(Alvarez et al., 2001). In another series of studies and using
bioactivity-directed isolation procedures, five new spirostan
saponins and two sterol glycosides were isolated. The structure
of the new saponins designated as SC-2 SC-6 (Figure 2) was
established, based upon spectroscopic measurements, especially
ID and 2D NMR data referring to their peracetate derivatives (Zamilpa
et al., 2002). All the isolated compounds were tested against
dermatophytes in culture (T. mentagrophytes and
T. rubrum) and all manifested antifungal activity. The most
active compound was shown to be SC-2 (MIC values of 12.5 µg L-1
each) followed by SC-4 (MIC values of 25 and 50 µg mL-1
against T. mentagrophytes and T. rubrum
respectively (Zamilpa et al., 2002).

|
|
R1 |
R2 |
|
SC-1 |
Qui(1®6)-Glc(1®6)-Glc |
H |
|
SC-2 |
Xyl(1®3)-Qui |
H |
|
SC-3 |
Xyl |
H |
|
SC-4 |
Qui |
H |
|
SC-5 |
Rha(1®3)-Qui |
H |
|
SC-6 |
Rha(1®3)-Qui |
OH |
Figure 2.
Chemical structure
of saponins SC-1 SC-6 from Solanum chrysotrichum
CLINICAL STUDIES
In order to evaluate
the effectiveness of plant extracts in humans, a pilot clinical
study was carried out, using extracts from leaves of S.
chrysotrichum among patients with tinae pedis
who attended the Regional Hospital of the Mexican Social
Security Institute in Cuernavaca, Morelos, Mexico. A group of 18
ambulatory patients was selected and treated with a cream
containing 5% of the methanolic extract of the leaves, which was
applied topically during 4 weeks of treatment, and these were
then compared with a similar number of infected patients that
were treated with miconazole. The results showed that after one
week, 42% of the patients from the group receiving the plant
extract recovered, while no cure was observed among those
receiving miconazole during this early period. Remission of
symptoms was observed among both groups when each treatment was
completed (García-Cruz, 1988). In a controlled and randomized
clinical investigation conducted recently, the effectiveness and
tolerability of a standardized phytodrug prepared from S.
chrysotrichum was tested among 101 patients diagnosed with
tinae pedis. A standardized solution was prepared from
saponin SC-2, obtained from the plant and applied to the
experimental group, while 2% ketoconazole was administered to
the control group. Both treatments were applied topically during
a period of four weeks. After the treatment, the results showed
a clinical effectiveness of 96% in the experimental group, and
92% for the ketoconazole group, with good tolerability (100%) in
the case of both groups (Herrera-Arellano et al., 2003).
BIOTECHNOLOGICAL
INVESTIGATIONS
Micropropagation and
Callus Formation
Due to the fact that
S. chrysotrichum grows in a restricted area of Chiapas
Mexico, and is currently threatened by over-harvesting, we
established the micropropagation of this species, as well as the
development of calluses, using axillary buds. Explants were
grown in Murashige and Skoogs (MS) medium, supplemented with
various growth regulators. Induction of rooted plants was
initiated, only when indol-3 acetic acid (IAA) was present as an
auxin in combination with either of two cytokinins: kinetin (KN)
or benzyladenine (BA); however, the combination of IAA (0.1 mg L-1)
+ BA (0.2 mg L-1) was found to be best suited to the
purpose of morphogenesis. Adaptation among in vitro-derived
rooted plants was high (94%), and twelve months after adapting,
the plants flowered (Villarreal and Muñoz, 1991).
Micro-propagated plants have been used as a source of raw
material in order to carry out chemical and pharmacological
studies.
Cell Suspension
Cultures
Once the first
bioactive saponin SC-1 was isolated and elucidated and with the
aim of producing high and controlled levels of the antifungal
compound, we decided to establish in vitro cell culture
systems for S. chrysotrichum. Initially, we developed
cell suspension cultures from friable calluses that were
cultivated in MS medium, in combination with 0.1 mg L-1
naphtalene acetic acid (NAA) + 0.2 mg L-1 KN
(Villarreal and Muñoz, 1991). The suspensions were established
in 100 mL MS media (250 mL Erlenmeyer flasks), supplemented with
2 mg L-1 KN, and with four different auxins; MS1 [2
mg L-1 2,4 dichlorophenoxyacetic acid (2,4-D)], MS2
(0.5 mg L-1 NAA), MS3 (1.3 mg L-1 IAA) and
MS4 [1 mg L-1 2,4,5- trichlorophenoxyacetic acid
(2,4,5-T)]. The flasks were incubated in a batch mode at 130 rpm
during 25 days of culture, and maintained at 28 + 2oC
with a daily photoperiod of 16 h, and with a light intensity of
approximately 25 µmol m-2 s-1 (Villarreal
et al., 1997a). The kinetic parameters concerning growth and
metabolite production were measured every 5 days (three
replicates): dry weight (DW), fresh weight (FW), pH, cell
viability, medium carbohydrates and SC-1 concentration.
Identification and quantification of SC-1 were carried out by
HPLC analysis, using an R1-71 Merck refractive index detector, a
Lichrosfer Si60 258 µm 4 mm, and a 5 mm Merck column; with a
mobile phase of methanol:chloroform:water (29:70:1), and a flow
rate of 1.2 mL min-1. Retention times of SC-1 peaks
(2.20 min) from cell cultures and wild plant material were
compared using co-chromatography. Authentication of SC-1 in the
cultures was corroborated by applying infrared to the in
vitro spectra and comparing this with that obtained from
wild plants. Cell growth was registered in the four types of
media employed; however, MS1 was selected, because a finer and
more homogeneous suspension was obtained. The effects of
inoculum size and sucrose concentration on the biomass
accumulation and synthesis of the active metabolite were
studied. The maximum cell biomass was 12.9 g DW L-1,
which represents a 5.6-fold increase over the inoculum. The
specific growth rate (µ) was 0.15 d-1. The maximum
concentration of SC-1 was 14.6 mg DW g-1
(representing fifty times that of field grown plants) which was
reached after 20 days using a 2% inoculum, complete MS1 medium
and sucrose, consisting of between 30 and 45 g L-1.
The culture reached stationary phase after 10 days, even though
a high level of sugar (ca. 22 g L-1) still remained
in the medium. Doubling times, based on fresh and dry weights
were 4.5 and 5.0 d respectively (Villarreal et al., 1997a).
Large-Scale Cell
Suspension Cultures
In order to obtain
higher biomasses and to increase productivity level, scale-up of
batch suspension cultures in bioreactors was investigated. Two
cell lines ccvx (cotyledon derived) and ccvz (hypocotyl derived)
of S. chrysotrichum were cultivated in 10 L airlift
bioreactors for a period of 3 to 4 weeks, using two inocula of 2
and 3 g DW L-1. A draw-fill batch culture mode was
also put to the test by harvesting 50% of the cell culture and
replacing this with fresh medium. The cell cultures grew in the
bioreactors, forming a homogeneous white to yellow suspension.
Batch growth and accumulation of SC-1 over a 21 day period in
culture, in the case of both cell lines, when using 2 g L-1
as inoculum showed a maximum biomass concentration of 14.1 and
5.9 g DW L-1 for ccvx and ccvz. Cell suspensions
manifested doubling times of 3.15 and 6.9 days respectively.
Accumulation of SC-1 in bioreactors was non-growth associated
and reached maximum values of 21 and 19 mg g-1 for
ccvx and ccvz (Villarreal et al., 1997b). Using 3 g L-1
of inoculum and the same culture conditions as described above;
maximum biomass concentrations reached 14.6 and 7.7 g DW L-1
for ccvx and ccvz, manifesting doubling times of 5.3 and 5.8 d,
respectively. Maximum SC-1 concentration for ccvx and ccvz were
23 and 20 mg DW g-1 after 17 and 24 d in culture
(Villarreal et al., 1997b). When a draw-fill batch culture mode
was introduced, a steady state of concentration of specific SC-1
was obtained, consisting of about 25 mg DW g-1,
during the second stage of the culture. The productivity reached
in the bioreactors was between 2.33 and 2.0 times higher than in
shake-flask cultures (Villarreal et al., 1997 b). These results
show that the use of draw-fill batch culture modes with S.
chrysotrichum cell suspensions is able to significantly
increase productivity, whilst eliminating dead periods such as
the time required to sterilize the bioreactor as well as initial
lag phases in the cultures.
Hairy Root Cultures
Once the new saponins
(SC-2 ─ SC-6) had been isolated and elucidated, a systematic
study was conducted among field cultivated plants, in order to
determine the content of the active principles, throughout the
year. The yield of the antifungal saponins, harvested from wild
and cultivated specimens is low and their accumulation in the
leaves fluctuates, depending on stationary and ontogenic
variables (Zamilpa et al., 2002). This situation prompted us to
initiate research aimed at inducing plant genotypes with the
capacity to express higher and controlled levels of the
antifungal compounds. It is well known that hairy root cultures
transformed using the soil born pathogen Agrobacterium
rhizogenes are considered a potentially valuable resource
for synthesizing and in some cases they also secrete an
important number of secondary metabolites. These systems exhibit
stable and fast growth rates, comparable to those found in cell
suspensions and also exhibit genetic and biochemical stability,
as well as producing a greater quantity of certain secondary
compounds (Flores et al., 1995; Yoshimato et al., 2003).
Transformed root
cultures of S. chrysotrichum were established by
infecting nodal segments with A. rhizogenes
C58C1/pRi15834 and A4/pRiA4pESC4 (Nieto, 2003). Root lines were
grown in solid B5 medium (Figure 3) and following five passages
they were cultured in liquid B5 nutrient medium without growth
regulators, showing the typical hairy roots phenotype over four
years of continuous sub-culturing.
Genetic
transformation of the cell line C58-431 was confirmed by PCR
analysis showing the integration of the rolA into the
plant genome (Nieto, 2003). The hairy root cell line was
cultivated in 250 mL flasks (100 mL of B5 medium), supplemented
with sucrose (30 g L-1) without hormones and
incubated at 26oC, under uniform conditions (8-10
µmol m-2 s-1), at 115 rpm in a gyratory
shaker. Forty day old batch cultures were established, and
growth (fresh and dry weight) and production of saponins were
evaluated every five days. Carbohydrate consumption (sucrose,
fructose and glucose) was analyzed by HPLC using an IR detector.
The column was of the NH2 type measuring 3.9 x 300
mm, with 125 A pore size and a 10 µm particle diameter from
Waters. Predetermined conditions were: mobile phase 20:80
acetonitrile: water with an operational flow of 1.5 mL min-1.
Identification and quantification of saponins SC2- SC6 was
carried out by HPLC analysis. Saponins representing the
standards were obtained from S. chrysotrichum wild
plants, as previously described (Zamilpa et al., 2002). Extracts
were analyzed on a Waters Delta prep 4000 modular HPLC system,
consisting of a U6K injector, 600E
pump system
controller and 9 Millenium 3.2 software), and a RI detector. The
analysis was carried out on two Chromolith TM RP-18 (100 x 4.6
mm, 2 µm) columns connected in series; the mobile phase was
35:65 acetonitrile:water at a flow rate of 1.5 mL min -1
for SC-2 and, 1.7 mL min -1 for SC-3 and SC-4. For
SC-5 and SC-6, the mobile phase was 37:63 acetonitrile:water at
a flow rate of 1.5 mL min-1 (Caspeta et al., 2005a).
After 40 days, the density of roots was 4.3 g DW L-1
which was 6 times greater than the inoculum. Conductivity of the
culture medium dropped in proportion to root tissue growth
(Figure 4a). Root growth followed first order kinetics with a
mean specific growth rate (µ) of 0.08 d-1. Sucrose
was hydrolyzed on day 20 when the exponential growth phase ended
(Figure 4b).
Production of the
active extract (a mixture of saponins extracted from chloroform)
in the roots was growth-associated. During the culture time,
only three of the saponins (SC-2, SC-3 and SC-4) were recovered
from root biomasses, exhibiting discontinuous patterns in their
peaking, but with differences in their accumulation profiles, so
that maximum yields for saponin SC-2 (0.37 mg DW g-1)
and SC-4 (0.851 mg DW g-1) were registered on day 15,
when saponin SC-3 was not present; while maximum yield for
saponin SC-3 (0.189 mg DW g-1) was recovered on day
10, when the levels of SC-2 and SC-3 had diminished (Figure 4c)
(Caspeta et al., 2005a). Saponins were not released into the
culture medium and SC-5 and SC-6 were not observed, either in
the biomass or in the culture medium. None of the saponin yields
extracted from hairy roots cultured in flasks was higher than
those observed for plant leaves.
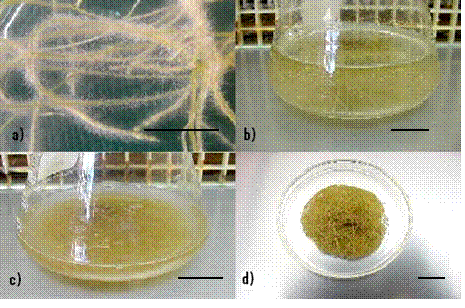
Figure 3.
Solanum
chrysotrichum
hairy root cultures: a) roots in solid medium b) roots growing
in liquid B5 medium c) after 15 days biomass, reaching 2 g FW L-1,
d) packet of root tissue.
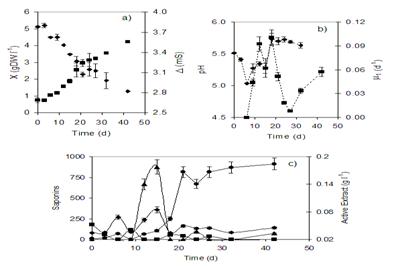
Figure 4.
Solanum
chrysotrichum
hairy root cultures in 250 mL flasks with 100 mL of B5 medium:
a) biomass X (n) and conductivity (u); (b) carbohydrate
consumption sucrose (), glucose(u) and fructose (·); (c) active
extract (·), SC-2(u), SC-3 (n) and SC-4 (). ( Reprinted with
permission from Caspeta et al (2005) Solanum chrysotrichum
hairy root cultures: characterization,scale-up, and production
of five antifungal saponins for human use. Planta Med 71:
1081-1084. Copyright (2008), Georg Thieme Verlag KG.
Large-Scale Hairy
Root Cultures
To scale-up the
growth and production of the hairy root cell line C58-431, 2 and
10 L airlift basic design reactors (BDRs) were used. The reactor
systems consisted of concentric draft-tube, internal-loop
airlift reactors (Figure 5) with a dD/dc
of 1.96 and hD/hc of
1.94. Gas sparging was carried out on the draft-tube bottom. The
downcomer had a cross-sectional area three times greater (Ad)
than the riser (Ar). Three adaptations for the BDR
were constructed and evaluated (Figure 5). A stainless steel
mesh draft, with the same downcomer diameter/column diameter
(dD/dC) and downcomer height/column
height (hD/hC) relationship, as
those on the BDR was introduced, and basic hydrodynamics were
maintained. Root tissue inoculation and distribution in the
downcomer was able to be promoted at low velocities, and growth
was promoted without any disruption of riser dynamics. A mesh
opening of 2.4 mm was chosen, in order to allow free root
enlargement from the downcomer to the riser and in order to
promote radial growth (Caspeta et al., 2005b) (Figure 5b). Other
fittings consisted of: a mesh draught tube with extensions
(Figure 5b) and mesh draught tube with extensions and helixes
(Figure 5c) (Caspeta et al., 2005b). Reactors were filled with a
sterilized B5 medium plus 3% sucrose, without growth regulators.
Small pieces of hairy
root tissues were suspended in 0.5 L of culture medium for
inoculation, using gravity. Tissue distribution in the downcomer
was performed at 0.001 vvm. In the case of the mesh-draft with
helixes, this structure was rotated, using 3.2 mm tube during
the inoculation process. Reactors were operated at 0.05 vvm for
3 days and then at 0.1 vvm until harvest on day 42, when draft
tubes were removed, and root beds were cut. In order to measure
homogeneity and distribution, downcomer and riser beds were
divided into three sections (Caspeta et al., 2005b). Root tissue
growth was indirectly monitored using conductivity measurements
as previously described (Tescione et al., 1997).

Figure 5.
Reactor and
modifications: a) basic design (concentric draft-tube
internal-loop airlift reactor) b) mesh draft tube, c) mesh draft
tube with extensions, d) mesh draft tube with extensions and
helixes. (Reprinted with permission from Caspeta et al., (2005)
Novel airlift reactor fitting for hairy root cultures:
developmental and performance studies. Biotechnology Progress
21:735-740. Copyright (2008), American Chemical Society.
Influence of tissue
geotropism was observed in the BDR. After some hours, roots got
trapped on the top and bottom draft extensions, and grew there
in dense tangles, manifesting necrotic tissue (Figure 6). After
42 days in culture, a maximum concentration of 2.04 g DW L-1
was obtained. During the time in culture, growth did not
appear to follow first-order kinetics (Caspeta et al., 2005b).
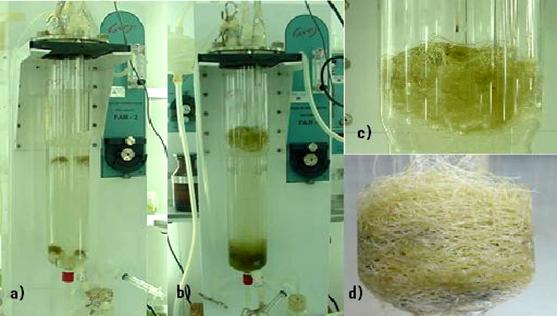    
Figure 6.
Hairy root culture of Solanum chrysotrichum in 2 L basic
design airlift bioreactors (BDR). One gram of FW L-1
was inoculated at the top and bottom of the bioreactor, a) poor
distribution of root tissue impaired homogeneous growth,
resulting in only 2.1 g DW L-1 of root biomass after
42 days in culture, b), c), and d).
Modifications on the
2 L reactor were undertaken (Figure 5). For distributing the
roots, the original glass draft tube was exchanged for a mesh
that allowed radial growth. The hydrodynamic patterns were
practically the same as in the BDR. At 0.01, vvm roots were
better distributed, although when the gas flow operation was
undertaken, roots moved to the arms for draft support as
happened in the BDR, so more arms were placed in the draft, and
when the gas flow operation was carried out, roots remained
distributed throughout the reactor. For a better suspension of
roots at the bottom of the reactor, helixes were placed on the
draft arms (Figure 5d) forming a perpetual screw, and their spin
helped in the movement of roots from the top to the bottom of
the reactor. With the last fitting roots were cultivated,
introducing inoculum by gravity to the downcomer and turning the
draft simultaneously to the right and left to distribute the
root tissue evenly. In the first two days, roots were deposited
in small packets on draft extensions, and after a few days,
roots started to grow along empty spaces between consecutive
arms, and a little radial growth was also observed. Mean
specific growth rate was 0.115 d-1, corresponding to
a doubling time of 6 days; which represented between 2 and 4
days less than that observed in the case of shake flasks and the
BDR, respectively. Roots were able to grow radially through the
mesh apertures, and on day 15, roots crossed the mesh draft and
began to fill the entire disposable area (Caspeta 2005b) (Figure
7).

Figure 7.
Hairy root culture of Solanum chrysotrichum in a 2 L
airlift modified bioreactor: a) after two days and b) after 45
days of culture.

Figure 8.
Hairy root tissue growing in 2 L airlift reactor modified with
mesh draft tube extensions and helixes, were harvested after 45
days: a) and b) lateral views, c) view from the bottom, d) and
e) cut off from growth at the middle of the root package.
After the culture
period (45 days), the biomass concentration doubled in
comparison with that obtained in the BDR. Roots were harvested
(Figure 8) and local root density was measured at the top (10.2
g DW L-1) middle (10.8 g DW L-1) and
bottom (11.88 g DW L-1), and no significant
differences were found after an arc-sine transformation of the
percentages, and the application of a Z test (p<0.13) (Caspeta
et al., 2005b). Considering only the disposable area for root
growth, a gross density of 8.7 g DW L-1 was obtained.
Accumulation of SC-2
(the most active saponin) in root biomasses grown in 2 L
reactors was of 7.17 mg DW g-1 (0.7% DW),
which was 19 and 6 times that of flask cultures or that obtained
from plant leaves, SC-4 yield was lower than the one from flask
cultures, and SC-3, SC-5 and SC-6 were not produced by
biomasses. In the culture medium, small concentrations of SC-5
and SC-6 were recovered (Caspeta et al., 2005a)
Table 1.
Yields of biomass and saponins in flasks and 2 L reactors of
Solanum chrysotrichum hairy roots
|
Volume |
Maximum biomass
(g DW L-1) |
Specific growth
rate (µ) d-1 |
Saponin content (mg DW g-1)
Biomass
Medium |
|
Flasks (100 mL) |
4.3 |
0.08 |
SC2 0.32 ± 0.05
nd
SC3 0.19 ± 0.03
nd
SC4 0.85 ± 0.12
nd |
|
Airlift reactor (2 L) |
4.4 |
0.11 |
SC2 7.17 ±1.4
nd
SC3 nd
nd
SC4 0.137 ± 0.03 nd
SC5 nd 0.028
± 0.012
SC6 nd 0.056
± 0.014 |
nd = not
detected
The scale-up of root
cultures from 2 to 10 L using modified reactors was evaluated.
The relationship between total draft area and total helix
longitude was maintained as in the 2 L modified reactor.
Therefore, the space between two consecutive helixes was twice
as long as the one in the 2 L. Inoculum pouring and
distribution were undertaken as in the 2 L. Root tips grounded
in the same way as those in 2 L, but little empty spaces were
observed and therefore root distribution was less homogeneous
than in the 2 L reactor (Figure 9).
Growth took place in
the downcomer as well as radially, inside the riser. After 20
days in culture, the growth kinetic was similar to that observed
in flasks and in the 2 L modified reactor (Caspeta et al.,
2005b). After 45 days in culture, a final biomass of 3.6 g DW L-1
was obtained.
CONCLUSIONS AND
OUTLOOK
Solanum chrysotrichum,
a plant species used to treat skin mycosis was selected in the
light of traditional medicinal knowledge, provided by Mayan
ethnic groups from Chiapas, Mexico. The pharmacological value of
this plant was demonstrated and we were able to isolate an
antifungal spirostanol saponin named SC-1, which represents a
new molecule. In a more recent study, five new bioactive
saponins (SC-2 ─ SC-6) were isolated and elucidated. Of these
compounds, SC-2 and SC-4 were shown to be the most efficacious
for eliminating dermatophytes in culture. Clinical studies
conducted among patients suffering from tinae pedis,
demonstrated the effectiveness and tolerability of a
standardized cream prepared with saponin SC-2, when compared
with a control group, treated with ketoconazole.
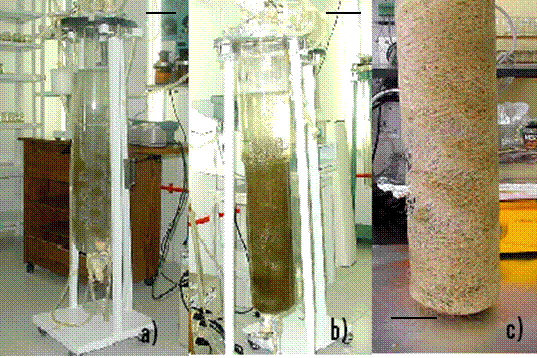
Figure 9.
Hairy root culture of Solanum chrysotrichum in 10 L
airlift modified bioreactor: a) root distribution following
inoculation, b) at 45 days, biomass reached 3.4 g DW L-1
and c) the pack of root tissue.
Even though S.
chrysotrichum is widely used as a popular medicine and grows in
a very limited region in Mexico, the plant has never been
cultivated, and the content of the antifungal compounds varies
seasonally and according to the age of the plant. The
biotechnological approaches described here may help promote the
permanent and controlled production of this antifungal medicine. The
plant was micropropagated, and the cell suspension cultures of this
species were scaled-up to 10 L, demonstrating that the airlift
bioreactors used for this purpose were adequate. Hairy root cultures
established in Erlenmeyer flasks were promising, as the most active
saponin SC-2 was produced with a maximum yield of 0.04% DW. Novel
fittings on 2 and 10 L airlift reactors were established to scale up
S. chrysotrichum hairy roots. The modified reactor with a
mesh replacing the glass draft tube and with additional extensions
and helixes exhibited improved root distribution, achieving the
highest root concentrations and manifesting more adequate dynamic
behavior. Moreover, the geometric scale-up of the fitting provided a
reproducible method for distributing inoculum, as well as an easier
method for harvesting and recovering biomass. Among root biomasses
grown in the reactor, the yield of SC-2 was 0.7% DW, a value
representing 19 and 6 times that from flask cultures or that
obtained from plant leaves. The results obtained with the hairy root
cultures of S. chrysotrichum offer feasible alternatives for
the production of bioactive saponins. These in vitro culture
procedures have made an important contribution towards establishing
the sustainable exploitation of this unique Mexican medicinal plant.
Thus the importance of these investigations becomes clear as this
species is transferred from the Maya highlands to the bioreactors
LITERATURE CITED
Alvarez L, Perez MC,
Gonzalez JL, Navarro V, Villarreal ML, Olson JO
(2001) SC-1 an
antimycotic spirostan saponin from Solanum chrysotrichum.
Planta Med 67: 372 - 374
Caspeta L, Nieto I,
Zamilpa A, Alvarez L, Quintero R, Villarreal ML (2005a)
Solanum chrysotrichum hairy root cultures: characterization,
scale-up and production of five antifungal saponins for human use.
Planta Med 71: 1081 - 1084
Caspeta L, Quintero R,
Villarreal ML
(2005b) Novel airlift reactor fitting for hairy root cultures:
developmental and performance studies. Biotechnol Prog 21:
735-40
García-Cruz M
(1988) Tratamiento de la
Tiña pedis con Solanum chrysotrichum Tesis, Facultad
de Medicina IMSS-IPN, Cuernavaca, Morelos
Flores HE, Medina-Bolivar
F (1995) Root
culture and plant natural products: unearthing the hidden half of
plant metabolism. Plant Tissue Cult Biotechnol 1: 59-74
Herrera-Arellano A,
Rodríguez-Soberanes A, Martínez-Rivera MA, MartÍnez-Cruz E, Zamilpa
A, Tortoriello J
(2003) Effectiveness and
tolerability of a standarized phytodrug derived from Solanum
chrysotrichum on Tinae pedis: a controlled and randomized
clinical trial. Planta Med 69: 390-395
Lozoya X, Aguilar A
(1987)
Encuesta sobre el uso actual de plantas en la Medicina Tradicional
Mexicana. Rev Med IMSSS (México) 25: 283-291
Lozoya X, Navarro V,
García M, Zurita M
(1991) Solanum chrysotrichum (Schdl). A plant used in Mexico
for treatment of skin mycosis. J Ethnopharmacol 36: 127-132
Nieto I
(2003) Establecimiento de
cultivos de raíces transformadas de Solanum chrysotrichum
para la producción de saponinas antifúngicas. Tesis de Maestría,
Centro de Investigación en Biotecnología, Universidad Autónoma del
Estado de Morelos, México
Tescione LD, Ramakrishnan
D, Curtis WR
(1997) The role of liquid mixing and gas-phase dispersion in a
submerged, spargued root reactor. Enzyme Microb Technol 20:
207-213
Villarreal ML, Muñoz J
(1991) Studies on the medicinal properties of Solanum
chrysotrichum tissue culture. Callus formation and plant
induction from axillary buds. Arch Med Res 22: 128-133
Villarreal ML, Arias C,
Feria-Velasco A, Ramírez OT, Quintero R
(1997a) Cell suspension culture of Solanum chrysotrichum (Schldl.).
A plant producing an antifungal spirostanol saponin. Plant Cell
Tissue Organ Cult 50: 39 -44
Villarreal ML, Arias C,
Vega J, Feria AV, Ramírez OT, Nicasio
P, Rojas G, Quintero R (1997b) Large-scale cultivation
of Solanum chrysotrichum cells: production of the antifungal
saponin SC-1 in 10-L airlift bioreactors. Plant Cell Rep 16:
653-656
Yoshimatsu K, Shimomura
K, Yamazaki M, Saito K, Kiuchi F
(2003) Transformation of
Ipec (Cephaelis ipecacuanha) with Agrobacterium rhizogenes
Planta Med 69: 1018-1023
Zamilpa A, Tortoriello J,
Navarro V, Delgado G, Alvarez L
(2002) Five new steroidal saponins from Solanum chrysotrichum
leaves and their antimycotic activity. J Nat Prod 65: 1815-
1819
Zurita M, Zolla C
(1986) Enfermedades dermatológicas en la Medicina Tradicional de
México Bol. Oficina Sanitaria Panamericana 101: 339-344
Accepted for
publication: 7 October 2008
|